Polatuzumab: Redefining Targeted Therapies in B-Cell Lymphoma
What You Need to Know About Polatuzumab
What is Polatuzumab??
Polatuzumab vedotin is an antibody-drug conjugate targeting CD79b, a component of the B-cell receptor, used primarily in B-cell lymphomas like DLBCL.
What is the mechanism of action for Polatuzumab?
It delivers a cytotoxic payload directly to B cells by binding to CD79b, leading to selective cell death with reduced off-target effects.
What are the clinical applications of Polatuzumab?
Polatuzumab is used in combination therapies for relapsed/refractory DLBCL and is being explored for front-line treatment.
Is Polatuzumab safe?
It has a manageable safety profile, with common side effects including neutropenia, peripheral neuropathy, and fatigue.
What is Polatuzumab vedotin-piiq?
This is the full brand name under which Polatuzumab was FDA-approved for clinical use.
1.) Understanding Polatuzumab
Polatuzumab vedotin has emerged as a transformative therapy in the landscape of antibody-drug conjugates (ADCs), particularly for B-cell lymphomas. By specifically targeting CD79b, a B-cell receptor component highly expressed in malignant B cells, Polatuzumab vedotin achieves precise delivery of its cytotoxic payload while sparing most healthy cells. This targeted mechanism of action significantly reduces systemic toxicity, a notable advantage over conventional chemotherapy.
The drug is composed of a monoclonal antibody linked to monomethyl auristatin E (MMAE), a potent microtubule-disrupting agent. Upon binding to CD79b, Polatuzumab vedotin is internalized by the cancer cell, where the linker is cleaved, releasing MMAE. This targeted cytotoxic action induces apoptosis in the malignant B cells, disrupting tumor proliferation and enhancing therapeutic outcomes.
Approved under the trade name Polivy (Polatuzumab vedotin-piiq), the ADC has shown substantial efficacy in patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL), particularly when used in combination with rituximab and bendamustine. Clinical trials have demonstrated that patients receiving the Polivy-based regimen achieve significantly higher response rates and longer progression-free survival compared to standard therapies.
Beyond DLBCL, Polatuzumab vedotin holds promise for other B-cell malignancies, given the widespread expression of CD79b across these cancers. Research is ongoing to assess its efficacy in combination with emerging agents and targeted therapies, aiming to further optimize treatment outcomes and reduce resistance. As interest in targeted immunotherapies continues to grow, Polatuzumab vedotin remains a critical focus for clinicians and researchers seeking to refine B-cell lymphoma management.
2.) Mechanism of Action of Polatuzumab
Polatuzumab vedotin leverages its antibody-drug conjugate (ADC) design to deliver targeted cytotoxic therapy to B-cell malignancies while minimizing off-target effects. The drug’s structure comprises a monoclonal antibody that specifically targets CD79b, a B-cell receptor subunit highly expressed on the surface of malignant B cells but largely absent in non-hematopoietic tissues. This targeted approach ensures that the cytotoxic payload is selectively delivered to cancerous cells, reducing systemic toxicity.
The payload, monomethyl auristatin E (MMAE), is a potent microtubule inhibitor that disrupts cellular division. Upon binding to CD79b, the Polatuzumab-MMAE complex is internalized through receptor-mediated endocytosis. Once inside the cell, the linker is cleaved, releasing MMAE directly into the cytoplasm. MMAE binds to tubulin and prevents its polymerization, effectively disrupting microtubule formation. This disruption induces cell cycle arrest and initiates apoptotic pathways, leading to selective cell death in the targeted B-cell population.
A critical advantage of Polatuzumab’s ADC design is its ability to deliver a cytotoxic agent directly to malignant cells while sparing healthy tissues. Unlike traditional chemotherapy, which indiscriminately affects rapidly dividing cells, Polatuzumab’s mechanism ensures that MMAE is activated only within B cells expressing CD79b. This specificity reduces the risk of systemic toxicity, including neurotoxicity and myelosuppression, common side effects associated with MMAE.
The drug’s efficacy is further amplified in combination regimens. Polatuzumab vedotin in combination with bendamustine and rituximab (BR) has shown promising results in patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL). Rituximab targets CD20, a surface antigen on B cells, while bendamustine is a chemotherapeutic agent with alkylating and antimetabolite properties. Together, these agents work synergistically to enhance the cytotoxic impact of Polatuzumab, improving overall response rates and progression-free survival in heavily pretreated patients.
As a targeted therapy, Polatuzumab vedotin exemplifies the evolving landscape of ADCs, offering a more precise and potentially less toxic therapeutic option for patients with aggressive B-cell lymphomas. Ongoing clinical trials are exploring its efficacy in earlier treatment lines and in combination with novel immunotherapies, positioning it as a cornerstone in modern lymphoma treatment strategies.
3.)Clinical Applications of Polatuzumab
Polatuzumab vedotin has become an essential component of treatment strategies for diffuse large B-cell lymphoma (DLBCL), particularly for patients who have exhausted other therapeutic options or are not eligible for stem cell transplantation. Its most prominent clinical application is in combination with bendamustine and rituximab (BR), targeting patients with relapsed or refractory DLBCL. This combination therapy received FDA approval following the pivotal GO29365 trial, which demonstrated that adding Polatuzumab to BR significantly improved response rates and overall survival compared to BR alone.
The GO29365 trial marked a significant advance in the management of DLBCL, as it addressed a critical unmet need for patients who had limited therapeutic options. The study’s results highlighted the enhanced efficacy of Polatuzumab when used with BR, showing a complete response rate of 40% in the Polatuzumab-BR group versus 18% in the BR-only group. These findings established Polatuzumab vedotin as a viable option for patients ineligible for autologous stem cell transplantation (ASCT) or those who have relapsed after multiple lines of therapy.
While initially positioned as a later-line treatment, Polatuzumab is now being investigated for earlier use. Emerging data from the POLARIX study supports its integration into first-line treatment regimens. In this study, Polatuzumab vedotin was combined with cyclophosphamide, doxorubicin, and prednisone (P-CHP), and compared to the standard R-CHOP regimen (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone). The P-CHP combination demonstrated superior progression-free survival (PFS), offering a potential new standard of care for previously untreated DLBCL patients.
The potential of Polatuzumab extends beyond DLBCL. Ongoing trials are evaluating its efficacy in other CD79b-positive malignancies, including follicular lymphoma. Given the widespread expression of CD79b in B-cell malignancies, there is a rationale for expanding its use across different lymphoma subtypes. Researchers are also exploring combination regimens that integrate Polatuzumab with novel agents, including bispecific antibodies and checkpoint inhibitors, aiming to enhance therapeutic outcomes through synergistic mechanisms.
Furthermore, advances in precision medicine are paving the way for personalized approaches to Polatuzumab therapy. Studies assessing molecular subtypes of DLBCL, such as cell-of-origin (COO) profiling, may help identify patient groups that derive the most benefit from ADC-based strategies. This personalized approach is crucial for optimizing efficacy and minimizing unnecessary toxicity.
Polatuzumab vedotin’s expanding role underscores its impact on modern lymphoma care. From later-line to frontline settings, and from DLBCL to potential applications in other B-cell malignancies, this ADC represents a paradigm shift in targeted oncology. As clinical evidence continues to accumulate, Polatuzumab is poised to remain at the forefront of B-cell lymphoma treatment innovation.
4.) Exploring Biosimilars for Polatuzumab
What is a Biosimilar?
A biosimilar is a biologic product that is highly similar to an approved reference biologic, with no clinically meaningful differences in safety, purity, or potency. Biosimilars are crucial for expanding access to biologics and enabling broader research opportunities.
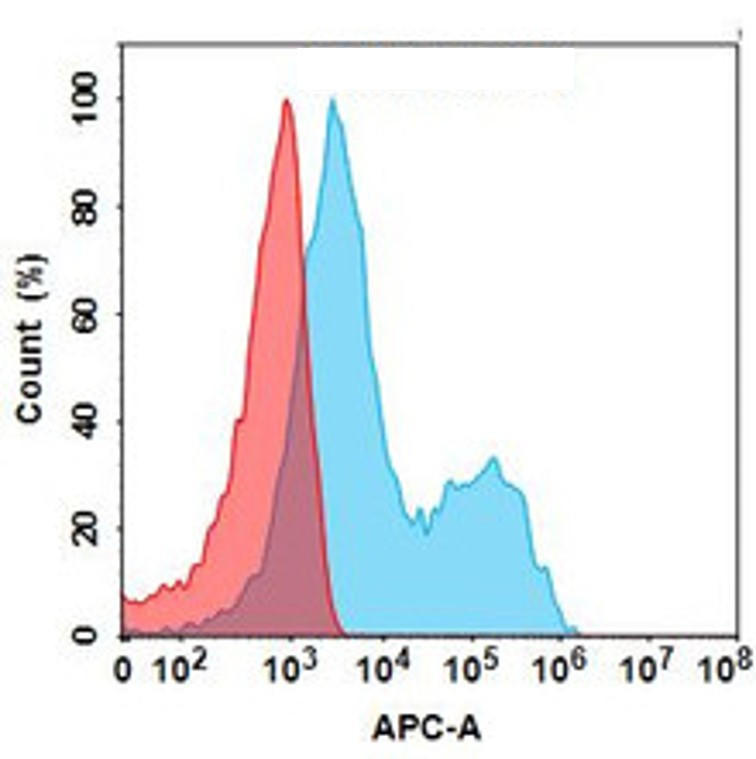
| Polatuzumab (Anti-CD79b) Biosimilar Antibody | |
|---|---|
| Antibody Type: | Monoclonal Antibody |
| Protein: | CD79B |
| Reactivity: | Human |
How Polatuzumab Biosimilar Compares to Polatuzumab
Our biosimilar to Polatuzumab vedotin offers structural and functional equivalence to the original molecule, targeting the same CD79b antigen. While not approved for clinical use, it is validated for non-clinical, investigational applications.
Advancing Research on Polatuzumab
- Validating CD79b-targeted pathways.
- Developing combination strategies.
- Screening ADC payloads in vitro.
- Supporting high-throughput analysis in preclinical pipelines.
Research Use Only Disclaimer:
This biosimilar is strictly for research use only and not for human or therapeutic use. It helps address the unmet need for accessible, reliable research-grade materials that enable deeper exploration of Polatuzumab's biology.
Discover Our Biosimilar Range
At Assay Genie, we specialize in providing high-quality biosimilars for research use! Check out our full biosimilar range to learn more.

By David Lee, PhD
Recent Posts
-
IgG1 Plasma Cells: The Emerging Biomarker for Predicting Cancer Immunotherapy Success
In the relentless fight against cancer, immunotherapy has emerged as a beacon of hope, harnessing t …24th Feb 2026 -
The Rise of Cancer Neuroscience: How Neural Circuits Drive Tumor Progression
For decades, we viewed cancer as a rogue army of cells, a biological glitch driven solely by geneti …23rd Feb 2026 -
CRISPR-Powered Light Sensors: A New Frontier in Ultra-Sensitive Cancer Detection
Cancer detection often relies on advanced imaging or invasive procedures, frequently catching the d …20th Feb 2026



