Enhancing Cancer Research: Syngeneic Mouse Models and Immune Checkpoint Inhibitors
Enhancing Cancer Research: Syngeneic Mouse Models and Immune Checkpoint Inhibitors
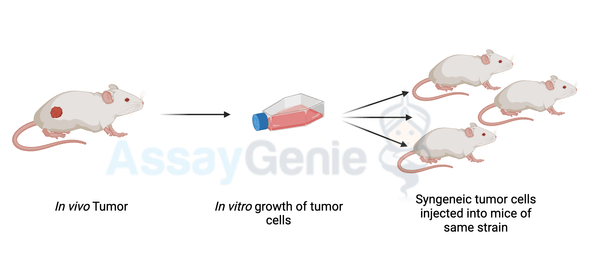
A quick guide to Syngeneic Mouse Models
Syngeneic Mouse Models
Cancer research has seen remarkable advancements in recent years, largely due to the development of novel therapeutic approaches such as immune checkpoint inhibitors (ICIs). Among the various preclinical models used in cancer research, syngeneic mouse models have emerged as indispensable tools for studying the tumor microenvironment and evaluating immunotherapeutic strategies. In this article, we will delve into the significance of syngeneic mouse models in cancer research, particularly focusing on their role in the study of immune checkpoint inhibitors.
Understanding Immune Checkpoints
Immune checkpoints are regulatory molecules that maintain immune homeostasis by modulating the intensity and duration of immune responses. While these checkpoints are crucial for preventing autoimmunity, cancer cells often exploit them to evade immune surveillance. ICIs work by blocking inhibitory signals mediated by checkpoint molecules, thereby reinvigorating antitumor immune responses. Key players in this therapeutic landscape include:
- PD-1/PD-L1 Axis: The interaction between PD-1, expressed on activated T cells, and its ligands PD-L1/PD-L2, expressed on tumor cells and immune cells, inhibits T cell function and promotes immune evasion by tumors.
- CTLA-4: CTLA-4 competes with the co-stimulatory molecule CD28 for binding to B7 ligands on antigen-presenting cells (APCs), thereby downregulating T cell activation and proliferation.
- Additional Checkpoints: Beyond PD-1/PD-L1 and CTLA-4, emerging checkpoint molecules such as lymphocyte activation gene 3 (LAG-3), T cell immunoglobulin and mucin-domain containing-3 (TIM-3), and T cell immunoreceptor with Ig and ITIM domains (TIGIT) offer promising targets for immunotherapy.
CTLA-4 competes with the co-stimulatory molecule CD28
The Role of Syngeneic Mouse Models in ICI Research:
Syngeneic mouse models serve as faithful surrogates for studying immune-tumor interactions and evaluating ICI efficacy in an immunocompetent setting. Their advantages in ICI research are manifold:
- Mimicking Tumor Microenvironment: Syngeneic models faithfully recapitulate the complex tumor microenvironment, including immune cell infiltration, stromal components, and cytokine milieu, providing a platform to dissect the interplay between ICIs and the immune system.
- Assessing Efficacy and Mechanisms of Action: By employing syngeneic models, researchers can elucidate the mechanisms underlying ICI efficacy, including tumor antigenicity, T cell priming, exhaustion, and memory formation. This insight is crucial for optimizing treatment strategies and overcoming resistance mechanisms.
- Personalized Medicine: Syngeneic models allow for the evaluation of ICI responses in the context of specific genetic backgrounds, tumor mutational burdens, and immune phenotypes, facilitating personalized therapeutic approaches.
- Predictive Biomarkers: Utilizing syngeneic models, researchers can identify predictive biomarkers of ICI response, such as PD-L1 expression, tumor mutational burden, T cell infiltration, and immune gene signatures, guiding patient selection and treatment decisions.
Case Studies:
Several preclinical studies using syngeneic mouse models have demonstrated the efficacy of ICIs across various cancer types:
- Breast Cancer: Syngeneic models of breast cancer have provided insights into the role of PD-L1 expression and tumor-infiltrating lymphocytes (TILs) in predicting response to PD-1/PD-L1 blockade. Combination therapies targeting multiple checkpoints or incorporating chemotherapy have shown synergistic effects in preclinical studies.
- Colorectal Cancer: Syngeneic models of colorectal cancer have highlighted the importance of the gut microbiota in modulating ICI responses. Strategies to modulate the microbiome or augment antigen presentation pathways have shown promise in enhancing ICI efficacy in preclinical models.
- Pancreatic Cancer: Despite initial challenges, syngeneic models of pancreatic cancer have provided valuable insights into the immunosuppressive tumor microenvironment and potential therapeutic targets. Combination therapies involving ICIs, stromal-targeting agents, and immunomodulatory drugs are under investigation to overcome resistance mechanisms in pancreatic cancer.
Advantages of Syngeneic Mouse Models
Several preclinical studies using syngeneic mouse models have demonstrated the efficacy of ICIs across various cancer types:
- Breast Cancer: Syngeneic models of breast cancer have provided insights into the role of PD-L1 expression and tumor-infiltrating lymphocytes (TILs) in predicting response to PD-1/PD-L1 blockade. Combination therapies targeting multiple checkpoints or incorporating chemotherapy have shown synergistic effects in preclinical studies.
- Colorectal Cancer: Syngeneic models of colorectal cancer have highlighted the importance of the gut microbiota in modulating ICI responses. Strategies to modulate the microbiome or augment antigen presentation pathways have shown promise in enhancing ICI efficacy in preclinical models.
- Pancreatic Cancer: Despite initial challenges, syngeneic models of pancreatic cancer have provided valuable insights into the immunosuppressive tumor microenvironment and potential therapeutic targets. Combination therapies involving ICIs, stromal-targeting agents, and immunomodulatory drugs are under investigation to overcome resistance mechanisms in pancreatic cancer.
Advantages of Syngeneic Mouse Models
1. Closer mimic of human biology: Syngeneic mouse models are derived from the same genetic background as the organism they are being used to study, which means they are more similar to humans than other types of animal models. This makes them a valuable tool in understanding the mechanisms of cancer and the development of new treatments.
2. Tumor microenvironment: Syngeneic mouse models allow the study of the interactions between cancer cells and the microenvironment, which can be very informative in understanding the progression of the disease.
3. Relevance to human cancer: As these models are derived from the same genetic background as the organism they are being used to study, their tumors are more relevant to human cancer than other types of animal models.
4. Easier to handle: Syngeneic mouse models are easy to handle and maintain, which makes them more practical for laboratory use.
Disadvantages of Syngeneic Mouse Models
1. Lack of genetic diversity: Because syngeneic mouse models are derived from the same genetic background, they lack the genetic diversity that is present in human populations. This can limit the applicability of the results to different human subpopulations.
2. Limited availability: Only a small number of syngeneic mouse models are available, which can limit the types of cancer that can be studied.
Advantages of Syngeneic Mouse Models
- Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015 Apr 9;161(2):205-14. doi: 10.1016/j.cell.2015.03.030. PMID: 25860605.
- Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018 Apr 27;359(6382):1350-1355. doi: 10.1126/science.aar4060. PMID: 29567705; PMCID: PMC6092597.
- Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, West AN, Carmona M, Kivork C, Seja E, Cherry G, Gutierrez AJ, Grogan TR, Mateus C, Tomasic G, Glaspy JA, Emerson RO, Robins H, Pierce RH, Elashoff DA, Robert C, Ribas A. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014 Nov 27;515(7528):568-71. doi: 10.1038/nature13954. Epub 2014 Nov 26. PMID: 25428505; PMCID: PMC4246418.
- Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017 Jan 18;541(7637):321-330. doi: 10.1038/nature21349. PMID: 28102259; PMCID: PMC5264572.
- Jenkins RW, Barbie DA, Flaherty KT. Mechanisms of resistance to immune checkpoint inhibitors. Br J Cancer. 2018 Jun;118(1):9-16. doi: 10.1038/bjc.2017.434. Epub 2018 Jan 16. PMID: 29334624; PMCID: PMC5765309.
- Gubin MM, Artyomov MN, Mardis ER, Schreiber RD. Tumor neoantigens: building a framework for personalized cancer immunotherapy. J Clin Invest. 2015 Apr;125(9):3413-21. doi: 10.1172/JCI80008. Epub 2015 Sep 1. PMID: 26325035; PMCID: PMC4588298.
- Homet Moreno B, Ribas A. Anti-programmed cell death protein-1/ligand-1 therapy in different cancers. Br J Cancer. 2015 Mar 31;112(9):1421-7. doi: 10.1038/bjc.2015.85. Epub 2015 Mar 17. PMID: 25783591; PMCID: PMC4453439.
Related Products
Written by Sean Mac Fhearraigh
Seán Mac Fhearraigh PhD is a co-founder of Assay Genie. Seán carried out his undergraduate degree in Genetics at Trinity College Dublin, followed by a PhD at University College Dublin. He carried out a post-doc at the Department of Genetics, University of Cambridge. Seán is now Chief Technical Officer at Assay Genie.
Recent Posts
-
Metabolic Exhaustion: How Mitochondrial Dysfunction Sabotages CAR-T Cell Therapy in Solid Tumors
Imagine engineering a patient's own immune cells into precision-guided missiles against cancer—cells …8th Dec 2025 -
The Powerhouse of Immunity: How Mitochondrial Fitness Fuels the Fight Against Cancer
Why do powerful cancer immunotherapies work wonders for some patients but fail for others? The answe …5th Dec 2025 -
How Cancer Cells Hijack Immune Defenses Through Mitochondrial Transfer
Imagine a battlefield where the enemy doesn't just hide from soldiers—it actively sabotages their we …5th Dec 2025