Protein Kinases: Overview, Classification and Therapeutic Potential
Kinases, key enzymes in cellular signaling, phosphorylate proteins to regulate crucial processes, and their modulation has significant implications in disease treatment.
Key Takeaways:
- Kinases are enzymes that phosphorylate proteins, influencing cell processes.
- They are categorized into serine/threonine, tyrosine, and dual specificity kinases.
- Kinases are crucial in cell signaling, growth, metabolism, and homeostasis.
- Dysregulation of kinases is linked to diseases like cancer and neurodegenerative disorders.
- Protein kinase inhibitors hold therapeutic potential in treating various diseases.
What are Kinases? Understanding their Functions, Types and Structure
Kinases are a pivotal class of enzymes instrumental in cellular signaling and regulation thanks to their ability to phosphorylate specific target molecules. Through the transfer of phosphate groups from ATP (adenosine triphosphate), kinases phosphorylate target proteins, modulating their activity and function. This process of phosphorylation serves as a fundamental mechanism by which kinases orchestrate crucial cellular processes, including cell growth and death, differentiation, metabolism, and intricate signal transduction pathways. By initiating a cascade of molecular events, kinases exert precise control over intricate cellular behaviors, playing a critical role in maintaining homeostasis.
Function of Protein Kinases
Protein kinases use adenosine triphosphate (ATP) as their source of energy for phosphorylation. When ATP binds to the catalytic domain of the enzyme, it is converted into adenosine diphosphate (ADP) and inorganic phosphate (Pi). The Pi then attacks the protein substrate, causing the transfer of a phosphate group from ATP to the protein.
Fig 1. Protein Kinase Function
Protein Kinase Classification
Protein kinases encompass a diverse array of enzyme types, each with its own unique characteristics and functional properties.Generally, these enzymes are categorized based on the presence of specific structural motifs that determine the amino acid residues that they phosphorylate on the substrate proteins. The three types of kinases are:
- Serine/Threonine Protein Kinases (STPKs)
- Tyrosine Kinases (TKs)
- Dual Specificity Protein Kinases (DSPKs)
Fig 2. Protein Kinase Classification
Serine/Threonine-Specific Protein Kinases (STPKs)
Serine/Threonine-specific Protein Kinases (STPKs) are a prominent type of protein kinase that catalyze the phosphorylation of serine and threonine residues in target proteins. They are involved in regulating a wide range of cellular processes, including cell growth, proliferation, differentiation, and apoptosis. Serine/threonine kinases are vital components of various signaling pathways, such as the transforming growth factor-beta (TGF-β) pathway and the mitogen-activated protein kinase (MAPK) pathway. They are further classified into classical STPKs, found in all eukaryotic cells, and atypical STPKs, found in only certain types of cells. Examples of serine/threonine kinases include protein kinase A (PKA), protein kinase C (PKC), and cyclin-dependent kinases (CDKs).
Tyrosine-Specific Protein Kinases (TKs)
Tyrosine kinases (Tks) are a distinct class of protein kinases that specifically phosphorylate tyrosine residues in target proteins. They play critical roles in cellular communication, proliferation, differentiation, and survival. They are further classifed into two types: Receptor Associated TKs, and non-receptor TKS. Receptor Associated TKs are found in the cytoplasm and are involved inreceptor signaling pathways, where ligand binding to receptor proteins triggers their activation. This leads to the phosphorylation of tyrosine residues in downstream signaling molecules, initiating various cellular responses. Non- receptor TKs are found in the nucleus and bind to DNA. Notable examples of tyrosine kinases include the epidermal growth factor receptor (EGFR), the insulin receptor, and the Src family kinases.
Dual Specificity Protein Kinases (DSPKs)
Dual-specificity kinases, as the name suggests, possess the ability to phosphorylate both serine/threonine and tyrosine residues. They exhibit versatility in their substrate specificity and often participate in intricate cellular processes. Dual-specificity kinases are involved in regulating cell cycle progression, DNA damage response, and cellular stress signaling. They are further classified into Type I , Type II and atypical DSPKs. Prominent examples include the mitogen-activated protein kinase kinase (MAP2K or MEK) family and the dual-specificity tyrosine phosphorylation-regulated kinases (DYRKs).
Protein kinases can be further classified according to their function. There are three main categories: signaling protein kinases, metabolic protein kinases, and housekeeping protein kinases. Signaling protein kinases play a role in signal transduction, metabolic protein kinases regulate metabolism, and housekeeping protein kinases perform essential cellular functions.
Structure of Protein Kinases
All protein kinases share a common structural motif, which is known as the catalytic domain. This domain contains the enzyme’s active site, where the phosphate groups are transferred from ATP to protein substrates. The catalytic domain is flanked by two other domains: the regulatory domain and the effector domain. The regulatory domain binds to proteins that regulate the activity of the enzyme, while the effector domain binds to proteins that are downstream of the enzyme’s action.
Protein Kinases in Signal Transduction
Protein kinases play a critical role in signal transduction, which is the process that allows cells to respond to external signals and regulate their activities. In particular, protein kinases can activate or inhibit other proteins by phosphorylating them. Phosphorylation allows cells to rapidly and reversibly change their gene expression in response to changes in their environment. This allows them to control many cellular processes, such as cell proliferation, cell death, and gene expression. Protein kinases are therefore essential for the proper functioning of cells.
Regulation of Protein Kinases
The regulation of protein kinases is a highly intricate process that ensures precise control over their activity and function. Various mechanisms contribute to the regulation of protein kinases, allowing for dynamic and context-dependent signaling. One common mode of regulation is through post-translational modifications, such as phosphorylation (which requires magneisum ions and is inhibited by compunds that compete with ATP for binding to enzyme), acetylation, or ubiquitination, which can either activate or inactivate kinases. Additionally, protein kinases can be subject to allosteric regulation, where binding of small molecules or other proteins modulates their activity. Activators increase the activity of protein kinases, while inhibitors decrease their activity. Ligands that bind to protein kinases can be either natural ligands (such as ATP) or synthetic ligands (such as drugs). Concentrations of ATP also influence Kinase activity.
Localization plays a crucial role as well, as kinases are often sequestered in specific cellular compartments or associated with scaffolding proteins to ensure spatial and temporal control of their signaling. Furthermore, feedback loops and cross-talk between signaling pathways further contribute to the regulation of protein kinases. By precisely orchestrating the regulation of protein kinases, cells can fine-tune their responses to external stimuli and maintain cellular homeostasis.
Diseases Associated with Unregulated Protein Kinase Activity
Protein kinases, as key regulators of cellular signaling pathways, play a crucial role in maintaining normal cell function. However, when these kinases become dysregulated, either through genetic mutations, alterations in expression levels, or aberrant activation mechanisms, they can contribute to the development and progression of debilitating diseases.Protein kinases, as key regulators of cellular signaling pathways, play a crucial role in maintaining normal cell function. However, when these kinases become dysregulated, either through genetic mutations, alterations in expression levels, or aberrant activation mechanisms, they can contribute to the development and progression of debilitating diseases.
1. Cancer: Dysregulation of protein kinase activity is a hallmark of cancer. Abnormal activation or overexpression of certain protein kinases can lead to uncontrolled cell growth, evasion of cell death, and tumor formation. For example, mutations in the gene that codes for c-Myc, a serine/threonine-specific protein kinase, can lead to the uncontrolled growth of cancer cells. Overactive protein kinases can promote tumor cell growth and survival. Other examples include the BCR-ABL kinase in chronic myelogenous leukemia (CML) and EGFR kinase in various solid tumors.
2. Neurodegenerative diseases: Protein kinase dysfunction has been implicated in neurodegenerative disorders such as Alzheimer's disease, Parkinson's disease, and Huntington's disease. Aberrant kinase activity can contribute to the accumulation of toxic protein aggregates, neuronal dysfunction, and cell death in the brain.
3. Cardiovascular diseases: Protein kinase dysregulation is involved in various cardiovascular conditions. For instance, abnormal activation of protein kinases like ERK1/2 and JNK can lead to cardiac hypertrophy, while dysregulation of kinases such as PKC and Akt can contribute to cardiac ischemia/reperfusion injury and heart failure.
4. Autoimmune disorders: In autoimmune diseases like rheumatoid arthritis and multiple sclerosis, dysregulated protein kinase signaling can contribute to the inappropriate activation of immune cells and the attack of healthy tissues. Kinases such as JAK and Syk are implicated in the pathogenesis of these diseases.
5. Metabolic disorders: Protein kinases play a role in metabolic regulation. Kinases such as AMPK and insulin receptor kinases are involved in the control of glucose and lipid metabolism and their dysregulation can contribute to metabolic disorders such as diabetes and obesity.
6. Developmental Disorders : During embryonic development, precise regulation of protein kinase signaling is crucial for orchestrating key processes such as cell proliferation, migration, differentiation, and organogenesis. Dysregulated protein kinase activity, arising from genetic mutations or disruptions in regulatory mechanisms, can perturb these delicate processes, leading to developmental abnormalities and congenital disorders. Examples include genetic mutations in kinases like MEK1/2, RAF, and FGFR, which are associated with developmental disorders such as Noonan syndrome, Apert Syndrome, Costello syndrome, and craniosynostosis syndromes. Furthermore, mutations in JAK kinases can lead to the development of immune deficiency diseases.
Fig 3. Diseases Associated with Protein Kinase Dysregulation and Potential Kinase Inhibitors for Therapy.
Protein Kinase Inhibitors and their therapeutic Potential
What are Protein Kinase Inhibitors?
Protein kinase inhibitors are a class of drugs that specifically target and block the activity of protein kinases. There are two main categories: small molecule inhibitors and peptide inhibitors. Small molecule inhibitors are used as standard chemotherapy drugs, while peptide inhibitors are in clinical trials.
Small molecule inhibitors are compounds that bind to protein kinases and inhibit their activity. These drugs include the chemotherapy drug gemcitabine, which inhibits the activity of the protein kinase PDK-I, and sunitinib malate, which inhibits the activity of several different protein kinases including VEGFR-II and c-Kit.
Peptide inhibitors are proteins that specifically bind to and inhibit the activity of protein kinases. These inhibitors include the peptide inhibitor PKI-1406, which binds to and inhibits the activity of the protein kinase PKC-δ, and AP20187, which binds to and inhibits the activity of several different protein kinases including ERK.
How do these inhibitors work?
Protein kinase inhibitors work by binding to the active site or other regions of the kinase, preventing its interaction with ATP and substrate proteins. This inhibition of kinase activity can disrupt signaling pathways and cellular processes that are dependent on the kinase's function. By selectively targeting specific protein kinases involved in disease pathways, these inhibitors offer the potential to modulate aberrant signaling and restore normal cellular function.
Protein kinase inhibitors are designed to be highly specific, targeting particular kinases based on their unique structural and functional characteristics. This specificity is crucial to minimize off-target effects and maximize therapeutic efficacy. Over the years, a wide range of protein kinase inhibitors have been developed, including tyrosine kinase inhibitors (TKIs), serine/threonine kinase inhibitors, and multi-kinase inhibitors. Each type of inhibitor targets a specific group of kinases or individual kinases known to be dysregulated in particular diseases.
Therapeutic Potential in Different Diseases
Protein kinase inhibitors have revolutionized cancer treatment, leading to significant advancements in precision medicine. They have become a cornerstone of targeted therapy, as they can specifically inhibit the kinases driving cancer cell growth and survival. Additionally, these inhibitors hold promise for treating other diseases characterized by dysregulated kinase signaling, such as autoimmune disorders, neurodegenerative diseases, and metabolic disorders. Some Examples are:
1. Cancer: Tyrosine kinase inhibitors, such as imatinib and gefitinib, have revolutionized the treatment of chronic myeloid leukemia (CML) and non-small cell lung cancer (NSCLC), respectively. Additionally, inhibitors targeting serine/threonine kinases, including BRAF and MEK inhibitors, have demonstrated significant clinical efficacy in treating melanoma harboring BRAF mutations. The development and optimization of protein kinase inhibitors continue to hold promise for precision medicine approaches in combating various types of cancer.
2. Neurodegenerative Diseases: Dysregulated protein kinase activity is implicated in the pathogenesis of conditions such as Alzheimer's disease, Parkinson's disease, and Huntington's disease. Inhibition of specific kinases involved in neurodegenerative processes, such as glycogen synthase kinase-3β (GSK-3β) or cyclin-dependent kinase 5 (CDK5), has shown promise in preclinical studies. These inhibitors can target aberrant kinase activity, modulate downstream signaling pathways, and potentially mitigate neuronal dysfunction and degeneration. While challenges remain in delivering kinase inhibitors to the brain, ongoing research efforts aim to harness the therapeutic potential of protein kinase inhibitors for treating neurodegenerative disorders.
3. Inflammatory and Autoimmune Disorders: Dysregulated kinase signaling plays a critical role in driving the inflammatory response and immune system dysregulation in conditions like rheumatoid arthritis, psoriasis, and inflammatory bowel disease. Inhibition of kinases, such as Janus kinases (JAKs), spleen tyrosine kinases (Syk), and Bruton's tyrosine kinase (BTK), has shown efficacy in clinical trials and gained regulatory approvals for specific indications. By targeting key kinases involved in the immune response, these inhibitors can modulate immune cell activation, cytokine production, and subsequent tissue damage, providing new avenues for treating inflammatory and autoimmune diseases.
Protein kinase inhibitors hold significant therapeutic potential across various disease areas. Their development and application are driven by a deeper understanding of the dysregulated kinase activity specific to each disease, allowing for targeted intervention and personalized treatment approaches.However, it is important to note that while protein kinase inhibitors have shown remarkable therapeutic potential, challenges remain. Drug resistance, off-target effects, and limited efficacy in certain patient populations are areas of ongoing research and improvement. Nevertheless, protein kinase inhibitors continue to be a promising area of drug development, offering new avenues for personalized and targeted therapeutic interventions in a variety of diseases.
Related Products
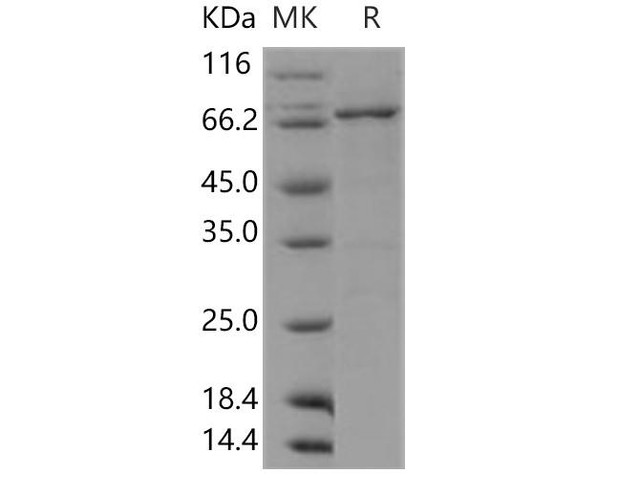
| Human DDR1 Kinase/MCK10 Recombinant Protein (RPES1613) | |
|---|---|
| Product Type | Recombinant Protein |
| Host Species | Baculovirus-Insect Cells |
| Reactivity | Human |
DDR1 is a receptor tyrosine kinase that belongs to the discoidin domain receptor family. It plays a critical role in cell adhesion, migration, extracellular matrix remodeling, and tissue homeostasis. Human DDR1 (Discoidin Domain Receptor 1) Kinase Recombinant protein is a valuable tool used in scientific research to investigate the biological functions and signaling pathways associated with DDR1 kinase.
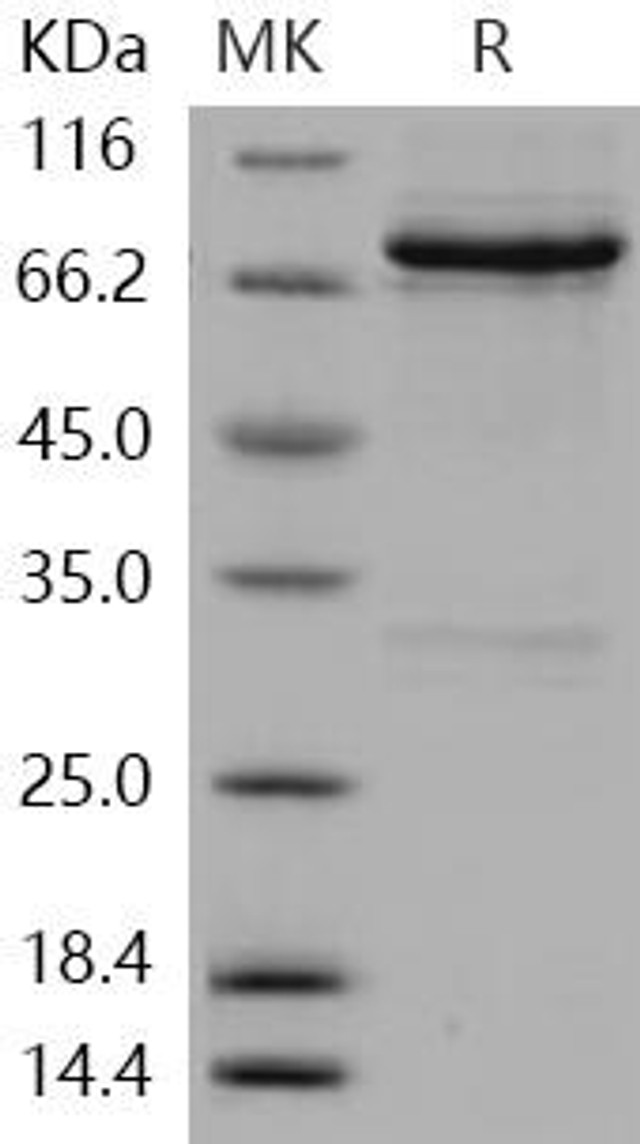
| Human RET Kinase Recombinant Protein (RPES0980) | |
|---|---|
| Product Type | Recombinant Protein |
| Host Species | Baculovirus-Insect Cells |
| Reactvity | Human |
The use of Human RET Kinase Recombinant protein enables researchers to gain insights into the molecular mechanisms, signaling cascades, and therapeutic potential associated with RET kinase.
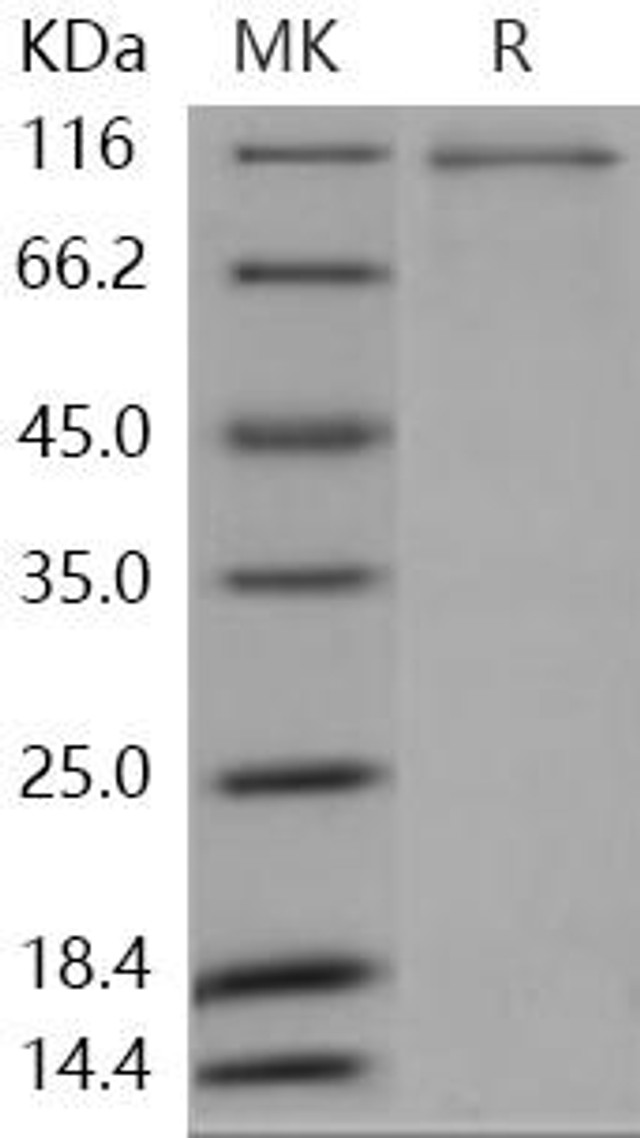
| Human MAP4K2/GC Kinase Recombinant Protein (RPES1066) | |
|---|---|
| Product Type | Recombinant Protein |
| Host Species | Baculovirus-Insect Cells |
| Reactivity | Human |
The use of Human MAP4K2 Kinase Recombinant Protein allows researchers to gain insights into the molecular mechanisms, signaling pathways, and potential therapeutic applications associated with MAP4K2 kinase.
Written by Rithika Suresh
Rithika Suresh completed her undergraduate degree in Biotechnology in Anna University before completing her masters in Biotechnology at University College Dublin.
Recent Posts
-
IgG1 Plasma Cells: The Emerging Biomarker for Predicting Cancer Immunotherapy Success
In the relentless fight against cancer, immunotherapy has emerged as a beacon of hope, harnessing t …24th Feb 2026 -
The Rise of Cancer Neuroscience: How Neural Circuits Drive Tumor Progression
For decades, we viewed cancer as a rogue army of cells, a biological glitch driven solely by geneti …23rd Feb 2026 -
CRISPR-Powered Light Sensors: A New Frontier in Ultra-Sensitive Cancer Detection
Cancer detection often relies on advanced imaging or invasive procedures, frequently catching the d …20th Feb 2026




