Deciphering the Intricacies of TGF-Beta Signaling Pathway
Transforming Growth Factor-Beta (TGF-β) signaling pathway plays a pivotal role in orchestrating various cellular processes, ranging from embryonic development to tissue homeostasis and immune response modulation. This intricate pathway is crucial for maintaining cellular balance, and dysregulation can contribute to a myriad of diseases, including cancer, fibrosis, and immune disorders. In this article, we delve into the key components and mechanisms that characterize the TGF-β signaling pathway.
Key Components of TGF-Beta Signaling Pathway:
TGF-β Ligands: The TGF-β family comprises multifunctional cytokines, with TGF-β1, TGF-β2, and TGF-β3 being the principal isoforms in mammals. These ligands are synthesized as precursor molecules and undergo proteolytic cleavage to form the active signaling molecules.
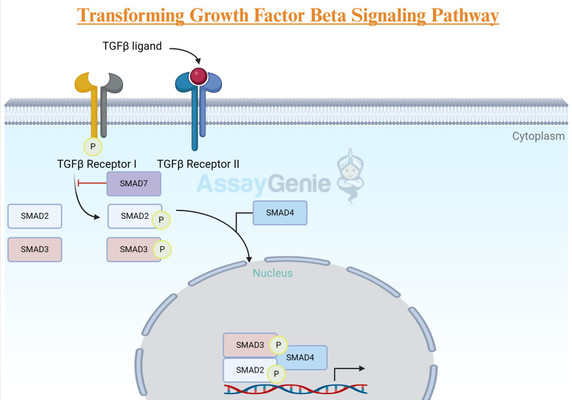
Receptors: The TGF-β receptors, known as TGF-β type I (TGF-βRI) and type II (TGF-βRII) receptors, are transmembrane proteins. TGF-βRII phosphorylates and activates TGF-βRI upon ligand binding, initiating downstream signaling.
Smad Proteins: Smads are key intracellular signaling molecules that relay the TGF-β signal from the cell membrane to the nucleus. Smad2 and Smad3 are activated by TGF-β and form a complex with Smad4, translocating to the nucleus where they regulate gene transcription.
The TGF-Beta Signaling Cascade:
Ligand Binding and Receptor Activation: TGF-β ligands bind to the extracellular domain of TGF-βRII, inducing a conformational change that enables TGF-βRII to phosphorylate TGF-βRI. This phosphorylation event activates TGF-βRI, initiating downstream signaling.
Smad Activation: Activated TGF-βRI phosphorylates Smad2 and Smad3, promoting their association with Smad4. This Smad complex then translocates to the nucleus, where it interacts with DNA-binding proteins to regulate gene transcription.
Gene Transcription Regulation: Within the nucleus, the Smad complex acts as a transcription factor, modulating the expression of target genes. TGF-β signaling can both activate and repress gene transcription, depending on the context and cellular milieu.
Cross-Talk with Other Signaling Pathways: The TGF-β pathway interacts with various other signaling cascades, including Wnt, Notch, and MAPK pathways, forming a complex network of regulatory cross-talk. This integration allows cells to precisely coordinate their responses to diverse signals.
Physiological Functions of the TGF-Beta Signaling Pathway:
Embryonic Development: TGF-β signaling is crucial for embryonic development, influencing cell differentiation, tissue patterning, and organ formation. Dysregulation of this pathway during development can lead to congenital abnormalities.
Cell Proliferation and Apoptosis: TGF-β acts as a potent regulator of cell proliferation, exerting both stimulatory and inhibitory effects depending on the cellular context. Additionally, TGF-β plays a role in apoptosis, ensuring the removal of damaged or unnecessary cells.
Immune Response: TGF-β is a key player in immune regulation, influencing the development and function of immune cells. It can act as both an immunosuppressive factor and a mediator of inflammation, depending on the immune microenvironment.
Tissue Repair and Fibrosis: TGF-β is essential for tissue repair and wound healing. However, persistent activation of the pathway can lead to excessive collagen deposition and fibrosis, contributing to pathological conditions in various organs.
Targeting TGF-β for Therapeutic Breakthroughs
References
Recent Posts
-
IgG1 Plasma Cells: The Emerging Biomarker for Predicting Cancer Immunotherapy Success
In the relentless fight against cancer, immunotherapy has emerged as a beacon of hope, harnessing t …24th Feb 2026 -
The Rise of Cancer Neuroscience: How Neural Circuits Drive Tumor Progression
For decades, we viewed cancer as a rogue army of cells, a biological glitch driven solely by geneti …23rd Feb 2026 -
CRISPR-Powered Light Sensors: A New Frontier in Ultra-Sensitive Cancer Detection
Cancer detection often relies on advanced imaging or invasive procedures, frequently catching the d …20th Feb 2026