Adipokines and Insulin Signaling Pathways: An In-Depth Exploration
The prevalence of obesity and its associated metabolic disorders has risen dramatically in recent decades, presenting a significant global health challenge. Adipose tissue, once considered merely a storage site for excess energy, is now recognized as an active endocrine organ secreting a myriad of bioactive molecules known as adipokines. Among these adipokines, a key player in metabolic regulation is insulin, a hormone produced by the pancreas.
Adipokines: Key Regulators of Metabolism
Adipokines function as critical mediators between adipose tissue and various organs, influencing metabolic processes such as glucose homeostasis, lipid metabolism, and inflammation. Leptin, adiponectin, resistin, and visfatin are prominent adipokines that modulate insulin sensitivity and contribute to the intricate network of signaling pathways.
Leptin
Leptin, predominantly produced by adipocytes, acts as a satiety signal in the hypothalamus, influencing energy balance and body weight. Studies have revealed that leptin also plays a role in insulin sensitivity by regulating insulin receptor substrate (IRS) phosphorylation and activation of phosphatidylinositol 3-kinase (PI3K)/Akt pathway.
Adiponectin
Adiponectin, another adipokine, exhibits insulin-sensitizing effects. It enhances glucose uptake and fatty acid oxidation in peripheral tissues while suppressing hepatic glucose production. Adiponectin achieves these effects by activating AMP-activated protein kinase (AMPK) and peroxisome proliferator-activated receptor-alpha (PPAR-α) signaling pathways.
Resistin
Resistin, secreted by adipocytes, was initially linked to insulin resistance. It modulates glucose metabolism by influencing the expression of insulin-responsive genes. Resistin exerts its effects through the activation of various signaling cascades, including c-Jun N-terminal kinase (JNK) and nuclear factor-kappa B (NF-κB).
Visfatin
Visfatin, also known as nicotinamide phosphoribosyltransferase (NAMPT), has been implicated in insulin-mimetic effects, promoting glucose uptake in adipocytes and skeletal muscle. Visfatin may influence insulin signaling through pathways involving insulin receptor substrate (IRS) and PI3K/Akt.
Insulin Signaling Pathways
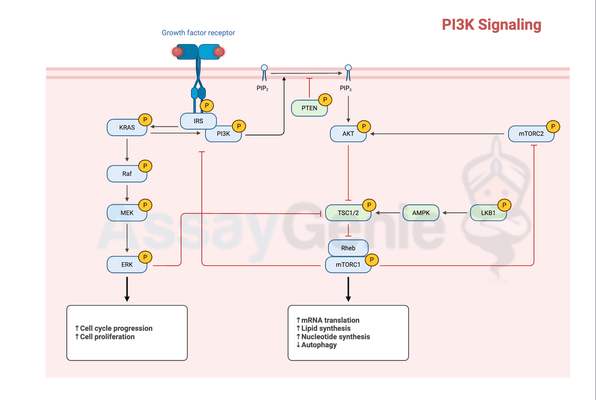
Insulin, a pivotal hormone in glucose homeostasis, engages in a complex network of signaling pathways to regulate cellular processes. The insulin signaling cascade primarily involves the activation of insulin receptor substrates (IRS) and subsequent activation of downstream effectors, including phosphoinositide 3-kinase (PI3K)/Akt and mitogen-activated protein kinase (MAPK) pathways.
Insulin Receptor Substrates (IRS)
Upon binding to its receptor, insulin initiates phosphorylation events on tyrosine residues of insulin receptor substrates (IRS). Phosphorylated IRS molecules serve as docking sites for downstream signaling molecules, transmitting the insulin signal to various cellular processes.
PI3K/Akt Pathway
One of the major insulin signaling pathways is the PI3K/Akt pathway, essential for glucose uptake and glycogen synthesis. Activation of PI3K leads to the generation of phosphatidylinositol-3,4,5-trisphosphate (PIP3), recruiting Akt to the cell membrane. Akt activation promotes glucose transporter 4 (GLUT4) translocation to the cell membrane, facilitating glucose uptake.
MAPK Pathway
The MAPK pathway, another branch of insulin signaling, regulates cell growth and differentiation. Insulin activates the Ras/Raf/MEK/ERK cascade, leading to the phosphorylation of various transcription factors and the modulation of gene expression involved in cell proliferation.
Crosstalk between Adipokines and Insulin Signaling
The intricate crosstalk between adipokines and insulin signaling pathways significantly impacts metabolic homeostasis. Dysregulation of this crosstalk can lead to insulin resistance and the development of metabolic disorders.
Leptin & insulin
Leptin, by modulating the PI3K/Akt pathway, enhances insulin sensitivity in target tissues. Leptin resistance, often observed in obesity, may contribute to impaired insulin signaling, promoting insulin resistance.
Adiponectin and Insulin
Adiponectin enhances insulin sensitivity by activating AMPK and PPAR-α pathways. Reduced adiponectin levels, commonly observed in obesity, are associated with insulin resistance and inflammation.
Resistin and Insulin
Resistin, through activation of JNK and NF-κB pathways, may interfere with insulin signaling. Elevated resistin levels are linked to insulin resistance, inflammation, and metabolic dysfunction.
Visfatin and Insulin
Visfatin's insulin-mimetic effects may contribute to enhanced glucose uptake. However, conflicting evidence exists regarding visfatin's role in insulin resistance, warranting further investigation.
Implications for Metabolic Disorders
Understanding the interplay between adipokines and insulin signaling pathways has important implications for the development of therapeutic strategies targeting metabolic disorders. Strategies aimed at modulating adipokine levels or improving insulin sensitivity may hold promise for mitigating obesity-related complications and type 2 diabetes.
Conclusion
In summary, adipokines and insulin signaling pathways orchestrate a complex interplay crucial for maintaining metabolic homeostasis. Leptin, adiponectin, resistin, and visfatin exert significant influences on insulin sensitivity through various signaling cascades. Elucidating the molecular mechanisms underlying this intricate crosstalk may unveil novel therapeutic targets for addressing metabolic disorders, contributing to advancements in the field of obesity and diabetes research.
References:
- Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006 Dec 14;444(7121):847-53.
- Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006 Dec 14;444(7121):840-6.
- Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011 Feb;11(2):85-97.
- Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001 Dec;414(6865):799-806.
- Scherer PE. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes. 2006 Jan;55(6):1537-45.
- Bluher M. Adipose tissue dysfunction in obesity. Exp Clin Endocrinol Diabetes. 2009 Nov;117(10):241-50.
- Ahlqvist E, Ahluwalia TS, Groop L. Genetics of type 2 diabetes. Clin Chem. 2011 Jul;57(7):241-54.
Recent Posts
-
Metabolic Exhaustion: How Mitochondrial Dysfunction Sabotages CAR-T Cell Therapy in Solid Tumors
Imagine engineering a patient's own immune cells into precision-guided missiles against cancer—cells …8th Dec 2025 -
The Powerhouse of Immunity: How Mitochondrial Fitness Fuels the Fight Against Cancer
Why do powerful cancer immunotherapies work wonders for some patients but fail for others? The answe …5th Dec 2025 -
How Cancer Cells Hijack Immune Defenses Through Mitochondrial Transfer
Imagine a battlefield where the enemy doesn't just hide from soldiers—it actively sabotages their we …5th Dec 2025