Vixarelimab: A Novel Approach to Targeting Prurigo Nodularis and Fibrosis
Quick Facts About Vixarelimab
What is Vixarelimab?
What is the mechanism of action for Vixarelimab?
What are the clinical applications of Vixarelimab?
1.) Understanding Vixarelimab
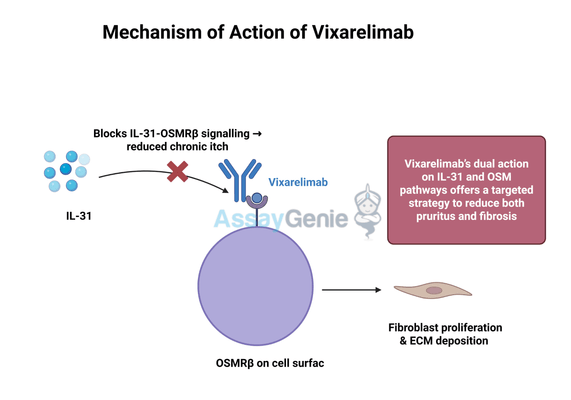
Vixarelimab is a first-in-class monoclonal antibody developed by Kiniksa Pharmaceuticals to block oncostatin M receptor beta (OSMRβ), a key receptor involved in chronic inflammation and fibrosis. This therapeutic approach has garnered attention for its potential in treating conditions such as prurigo nodularis (PN), a severe skin disorder characterized by persistent itching and nodular lesions, and idiopathic pulmonary fibrosis (IPF), a progressive lung disease that leads to scarring of lung tissue. By inhibiting the OSMRβ pathway, Vixarelimab aims to address the underlying mechanisms of these conditions rather than just alleviating symptoms, making it a promising disease-modifying agent.
Inflammation and fibrosis contribute to a wide range of chronic diseases, with the OSMRβ pathway playing a crucial role in regulating these processes. OSMRβ is a shared signaling receptor for oncostatin M (OSM) and interleukin-31 (IL-31), two cytokines involved in immune response, tissue remodeling, and fibrosis. Traditional therapies for prurigo nodularis and IPF focus primarily on symptom management rather than modifying disease progression. Vixarelimab, however, represents a novel approach by directly targeting the inflammatory and fibrotic pathways involved in these conditions.
Despite early promise, Vixarelimab's development path has undergone adjustments. Some clinical studies, particularly those related to fibrotic diseases, were discontinued due to trial results or strategic shifts in drug development. However, research into its efficacy for prurigo nodularis and other inflammatory disorders continues, highlighting the importance of OSMRβ inhibition as a therapeutic strategy. As studies progress, Vixarelimab could provide valuable insights into targeting chronic inflammation and fibrosis, potentially leading to more effective treatments for a range of dermatologic and pulmonary conditions.
2.) Mechanism of Action of Vixarelimab
Vixarelimab is a fully human monoclonal antibody that functions by selectively binding to OSMRβ, thereby blocking its interaction with two key cytokines: oncostatin M (OSM) and interleukin-31 (IL-31). These cytokines play a significant role in inflammation, pruritus (itch sensation), and fibrosis, making their inhibition a potentially effective strategy for treating chronic inflammatory and fibrotic diseases.
Pruritus Regulation
IL-31 is a well-known mediator of chronic itch, particularly in conditions such as prurigo nodularis and atopic dermatitis. When IL-31 binds to OSMRβ, it triggers a signaling cascade that stimulates nerve fibers responsible for itch sensation. This process leads to the persistent and intense itching that characterizes PN. By blocking OSMRβ, Vixarelimab prevents IL-31 from activating its pathway, reducing both itch severity and the development of skin lesions associated with chronic scratching.
Fibrosis Development
OSM is implicated in tissue remodeling and fibrotic progression, particularly in lung diseases like idiopathic pulmonary fibrosis. OSMRβ activation promotes fibroblast proliferation and extracellular matrix deposition, contributing to excessive scarring and organ dysfunction. By inhibiting OSMRβ, Vixarelimab disrupts this signaling pathway, potentially slowing or preventing fibrosis. This mechanism positions the drug as a promising candidate for fibrotic conditions where current therapies have limited efficacy.
Unlike conventional treatments that primarily manage symptoms, Vixarelimab’s dual mechanism of reducing both pruritus and fibrosis offers a unique therapeutic advantage. By directly targeting cytokine signaling, it provides a more targeted and potentially more effective approach to managing inflammatory and fibrotic diseases. Ongoing studies continue to explore its broader implications in immunology and dermatology, assessing its long-term benefits and safety in various patient populations.
Pruritus RegulationFibrosis Development3.) Clinical Applications of Vixarelimab
Prurigo Nodularis (PN)
Prurigo nodularis is a debilitating chronic skin disorder marked by extreme pruritus and the formation of thickened, hyperkeratotic nodules due to repetitive scratching. Current treatments, such as topical corticosteroids, antihistamines, and immunosuppressants, often provide only partial relief, leaving many patients with persistent symptoms. Vixarelimab has been developed as a targeted therapy for PN by blocking IL-31 signaling, which is a key driver of chronic itch and inflammation.
Clinical trials evaluating Vixarelimab for PN have shown promising results. Patients receiving the drug demonstrated significant reductions in itch intensity, leading to improved quality of life and decreased lesion count. Unlike traditional therapies that primarily suppress symptoms, Vixarelimab targets the root cause of PN by preventing IL-31 from activating nerve pathways responsible for persistent itching. This makes it a potentially transformative treatment for patients who have struggled with inadequate responses to existing therapies.
Idiopathic Pulmonary Fibrosis (IPF) and Other Fibrotic Conditions
Idiopathic pulmonary fibrosis is a severe, progressive lung disease characterized by excessive scarring (fibrosis) that impairs respiratory function over time. Current treatments, such as antifibrotic drugs, slow disease progression but do not halt or reverse fibrosis. The role of OSM in promoting fibroblast activity and extracellular matrix deposition has made OSMRβ an attractive target for antifibrotic therapy.
Preclinical and early clinical studies suggested that blocking OSMRβ with Vixarelimab might slow fibrosis progression by disrupting pro-fibrotic signaling. However, despite initial optimism, some clinical trials assessing Vixarelimab’s efficacy in IPF were discontinued due to inconclusive results or challenges in demonstrating significant clinical benefit. These findings highlight the complexity of targeting fibrosis and the need for further research into patient selection, combination therapies, and alternative dosing strategies to optimize outcomes.
Other Potential Applications
Beyond prurigo nodularis and idiopathic pulmonary fibrosis, Vixarelimab is being investigated for its potential role in treating other inflammatory and fibrotic diseases. Conditions such as atopic dermatitis, ulcerative colitis, and systemic sclerosis may also involve dysregulated OSMRβ signaling, making this receptor an interesting target for future research. While studies in these areas are still in early phases, Vixarelimab’s ability to modulate immune responses and inhibit fibrosis could expand its therapeutic utility across multiple disease states.
As research progresses, the drug’s long-term efficacy, safety, and broader applications will become clearer. The ability of Vixarelimab to target both inflammation and fibrosis uniquely positions it as a potential breakthrough therapy for multiple chronic diseases, offering hope for improved treatment options where current therapies remain inadequate.
Idiopathic Pulmonary Fibrosis (IPF) and Other Fibrotic ConditionsOther Potential Applications
4.) Exploring Biosimilars for Vixarelimab
What is a Biosimilar?
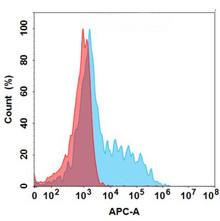
| Vixarelimab (Anti-OSMR) Biosimilar Antibody | |
|---|---|
| Antibody Type: | Monoclonal Antibody |
| Protein: | OSMR |
| Reactivity: | Human |
How Vixarelimab Biosimilar Compares to Vixarelimab
A biosimilar to Vixarelimab would provide a research-grade alternative for scientists exploring OSMRβ inhibition. While it is not interchangeable with the original drug for clinical use, it offers a valuable tool for:
- Preclinical studies on prurigo nodularis and fibrosis
- Understanding OSMRβ’s role in inflammation and tissue remodeling
- Exploring combination therapies for fibrotic diseases
Advantages of Vixarelimab Biosimilar in Research
Cost-Effective: Enables broader access for academic and industrial research.
Consistency: Maintains structural and functional similarity to the reference product.
Advancing Drug Discovery: Facilitates early-stage drug development and biomarker identification.
Research Use Only Disclaimer:
Discover Our Biosimilar Range
At Assay Genie, we specialize in providing high-quality biosimilars for research use! Check out our full biosimilar range to learn more.

By Chris McNally, PhD
Chris McNally, PhD, has a strong foundation in Biomedical Science, completing a PhD scholarship in collaboration with Randox Laboratories and Ulster University. Chris has published extensively in prostate cancer research, focusing on biomarker discovery, cancer risk stratification, and molecular mechanisms such as hypoxia-induced regulation. He currently serves as a Business Development Manager at Assay Genie.
Recent Posts
-
Metabolic Exhaustion: How Mitochondrial Dysfunction Sabotages CAR-T Cell Therapy in Solid Tumors
Imagine engineering a patient's own immune cells into precision-guided missiles against cancer—cells …8th Dec 2025 -
The Powerhouse of Immunity: How Mitochondrial Fitness Fuels the Fight Against Cancer
Why do powerful cancer immunotherapies work wonders for some patients but fail for others? The answe …5th Dec 2025 -
How Cancer Cells Hijack Immune Defenses Through Mitochondrial Transfer
Imagine a battlefield where the enemy doesn't just hide from soldiers—it actively sabotages their we …5th Dec 2025