Neuroimmunology: The Immune System of the CNS
What is Neuroimmunology?
Neuroimmunology is a multidisciplinary scientific field dedicated to investigating the intricate and bidirectional interactions between the central nervous system (CNS) and the immune system. It involves the rigorous study of regulatory influences and cross-talk that govern the functionality and behavior of these once-segregated systems.
The CNS
Immune Cells of the CNS
The CNS is not the isolated and immune-privileged entity it was once thought to be. Emerging research has highlighted the presence of a dynamic and intricate interaction between the CNS and the immune system, involving various immune cells and their effector functions. Understanding the roles of these immune cells and the cytokines they produce is critical in deciphering the complexities of neuroimmunology.
-
Routes of CNS Entry and Exit for Immune Cells:
Previously, the CNS was believed to lack lymphatic vessels and was considered immune-privileged. However, recent discoveries have challenged this notion. Lymphatic vessels have been found in the dural sinuses, carrying cerebrospinal fluid (CSF)-derived immune cells and fluid. Additionally, during conditions like ischaemic stroke, alternative pathways for immune cell entry into the CNS have been observed. Pro-inflammatory gdT cells have been found to secrete interleukin-17 (IL-17) and enter the ischaemic brain through the leptomeninges, while neutrophils are recruited via a breach in the blood-brain barrier (BBB) post-stroke. The route of immune cell entry appears to be influenced by the anti-inflammatory status of the infiltrating cell, as beneficial monocytes are recruited through the choroid plexus in certain contexts like stroke and spinal cord injury. -
Tissue-Resident Macrophages and Type 2 Innate Lymphoid Cells:
In addition to immune cells infiltrating the CNS during homeostasis and disease, the CNS harbors various tissue-resident macrophages, including microglia, perivascular macrophages, and meningeal macrophages. These specialized macrophages originate from the yolk sac during embryonic development and play pivotal roles in CNS immune surveillance and tissue homeostasis.Moreover, the meningeal space has been found to contain a population of type 2 innate lymphoid cells (ILC2). These ILC2 have demonstrated their significance in mediating tissue repair by producing type 2 cytokines in an IL-33-dependent manner post-injury. Such findings challenge the traditional notion of immune privilege within the CNS, indicating that immune cells are present in the CNS parenchyma and its frontiers, participating in both innate and adaptive immune responses. This immune privilege is susceptible to compromise during inflammatory conditions.
-
Effector Functions of Immune Cells in the CNS:
Once immune cells gain access to the CNS, they exert their effector functions, influencing the neuroimmune response. Microglia, as resident macrophages, act as the primary immune sentinels within the CNS, surveying the microenvironment and responding rapidly to changes. They can adopt either neuroprotective or pro-inflammatory phenotypes, depending on the signals they encounter.Upon activation, immune cells secrete cytokines, which are essential signaling molecules in the immune response. Cytokines like interleukins (e.g., IL-6, IL-1β), interferons (e.g., IFN-γ), and tumor necrosis factor-alpha (TNF-α) play critical roles in modulating immune responses within the CNS. These cytokines can either promote inflammation and tissue damage or contribute to tissue repair and resolution of inflammation. The balance between pro-inflammatory and anti-inflammatory cytokines is crucial in determining the outcome of CNS immune responses.
The Blood-Brain Barrier and Neuroimmunology
The blood-brain barrier (BBB) is a specialized, semipermeable interface between the circulatory system and the CNS. Composed of tightly sealed endothelial cells, pericytes, and astrocytic foot processes, the BBB restricts the passage of substances from the bloodstream into the brain, protecting it from potential harmful agents.
In the context of neuroimmunology, the BBB plays a pivotal role in regulating immune responses within the CNS. It selectively permits the entry of essential nutrients and molecules required for proper brain function while preventing the influx of pathogens, toxins, and immune cells from the systemic circulation. This tightly regulated barrier actively contributes to maintaining the CNS's immune privilege.
Despite its protective function, the BBB can be compromised during various neuroinflammatory conditions. In response to CNS injuries, infections, or autoimmune diseases, immune cells may breach the BBB and infiltrate the CNS, leading to neuroinflammation and further tissue damage.
The Neuroimmune System and Inflammation
Neuroinflammation, intricately linked with the neuroimmune system, plays a critical role in CNS diseases. When the CNS encounters threats or injuries, immune responses are triggered, involving activation of resident immune cells like microglia and astrocytes, and infiltration of peripheral immune cells through the compromised blood-brain barrier. Pro-inflammatory cytokines released by activated immune cells promote further immune cell recruitment, intensifying the inflammatory response.
While inflammation serves as a protective mechanism for tissue repair and pathogen clearance, uncontrolled or chronic inflammation can lead to harmful effects, damaging neuronal tissue and causing demyelination and neurodegeneration. Multiple sclerosis (MS) exemplifies neuroinflammation, driven by CNS-reactive T cells and autoantibodies targeting myelin and axonal proteins, resulting in chronic inflammation and demyelination. However, inflammation is a nuanced process, as specialized immune cell subsets and anti-inflammatory signals can also regulate the immune response, promoting tissue repair and regeneration.
Role of Neuroinflammation in Neurodegenerative Diseases
The Neuroimmune System in Disease
The blurring of the boundaries between the CNS and the immune system means that diseases affecting one system have an inevitable impact on the other. Diseases affecting systemic immunity such as infections or autoimmune disease such as MS can also significantly impact CNS function including the physical damage of neuronal networks as well as cognition. This occurs predominantly through the propagation of neuroinflammation as a consequence of both myeloid and lymphoid cells from the periphery infiltrating the CNS.
The Neuroimmune System and MS
MS arises from dysregulated immune responses against self-antigens, particularly myelin proteins in the CNS. A pivotal event involves the activation of CNS-reactive T cells. The process starts in the periphery, where autoreactive T cells are primed against myelin antigens presented by antigen-presenting cells (APCs). Escaping peripheral tolerance mechanisms, these activated T cells infiltrate the CNS, breaching the BBB.
Within the CNS, these CNS-reactive T cells recognize and interact with myelin antigens presented by resident APCs, contributing to ongoing inflammation and demyelination. The secretion of pro-inflammatory cytokines, such as interleukin-17 (IL-17) and interferon-gamma (IFN-γ), amplifies the immune response and recruits other immune cells, such as macrophages and B cells. Macrophages are activated to phagocytose myelin, leading to the characteristic demyelination and axonal damage seen in MS.
The self-perpetuating cycle of inflammation and tissue damage within the CNS culminates in the formation of MS lesions and the manifestation of neurological deficits in affected individuals.
Activation of CNS-Reactive T-cells in MS
The Neuroimmune System and Alzheimer's Disease
Alzheimer's disease (AD), a progressive neurodegenerative disorder, involves complex interactions between the neuroimmune system and the CNS. Inflammation plays a significant role in the pathogenesis of AD, and both resident immune cells like microglia and infiltrating immune cells from the periphery contribute to the neuroinflammatory response.
In AD, the brain's microglia become activated, releasing pro-inflammatory cytokines, such as interleukin-1 beta (IL-1β) and tumor necrosis factor-alpha (TNF-α), exacerbating neuroinflammation. Chronic inflammation in the brain can lead to the accumulation of amyloid-beta plaques and the formation of neurofibrillary tangles, characteristic features of AD pathology.
Additionally, immune responses in AD may also involve the adaptive immune system, with evidence of T cells and B cells infiltrating the brain and contributing to neuroinflammation. The intricate interplay between the immune system and the CNS in Alzheimer's disease underscores the potential of immunomodulatory therapies as promising avenues for treatment, aiming to regulate the inflammatory response and potentially slow disease progression.
Neuroimmunology Related Kits
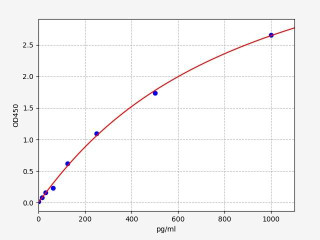
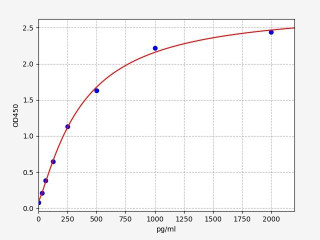
| Human TNF alpha ELISA Kit | |
|---|---|
| ELISA Type | Sandwich |
| Sensitivity | 9.375pg/ml |
| Range | 15.625-1000pg/ml |
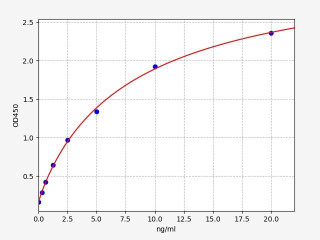
| Human AQP4 / Aquaporin-4 ELISA Kit | |
|---|---|
| ELISA Type | Sandwich |
| Sensitivity | 0.188ng/ml |
| Range | 0.313-20ng/ml |
Written by Lauryn McLoughlin
Lauryn McLoughlin completed her undergraduate degree in Neuroscience before completing her masters in Biotechnology at University College Dublin.
Recent Posts
-
IgG1 Plasma Cells: The Emerging Biomarker for Predicting Cancer Immunotherapy Success
In the relentless fight against cancer, immunotherapy has emerged as a beacon of hope, harnessing t …24th Feb 2026 -
The Rise of Cancer Neuroscience: How Neural Circuits Drive Tumor Progression
For decades, we viewed cancer as a rogue army of cells, a biological glitch driven solely by geneti …23rd Feb 2026 -
CRISPR-Powered Light Sensors: A New Frontier in Ultra-Sensitive Cancer Detection
Cancer detection often relies on advanced imaging or invasive procedures, frequently catching the d …20th Feb 2026