Growth Factors: Key Players in Biological Processes
Growth factors play a vital role in the complex web of biological processes, serving as key regulators in various essential events. They exert significant influence over crucial cellular behaviors such as proliferation, differentiation, and survival.
Acting as molecular messengers, growth factors facilitate communication between cells and tissues, ensuring coordinated functionality.
By exploring the defining characteristics and functions of growth factors, we gain a deeper understanding of their profound contributions to embryonic development, tissue repair, and cellular communication. Join us on this exploration as we delve into the world of growth factors and their crucial roles as key players in biological processes.
Table of Contents
Jump to a section:
Understanding Growth Factors: Definitions and Functions
The initial definition of Growth Factors was that of a secreted biologically active molecule that can affect the growth of cells. However, the definition has, over time, expanded to include secreted proteins/ peptides that modulate cell differentiation and survival.
Growth factors act as molecular messengers and through their binding on cell surface receptors, these potent agents trigger a cascade of intracellular events that ultimately govern cellular behaviors.
The multifaceted functions of growth factors extend beyond embryonic development and encompass tissue repair, wound healing, and maintenance of homeostasis in adult organisms.
An Overview of Growth Factor Signalling
Classification and Types of Growth Factors
Growth factors exhibit a diverse array of molecules, each playing a specific role in regulating cellular processes. Based on their structural and functional characteristics, growth factors can be classified into several distinct types. One common classification scheme groups growth factors into families based on similarities in their amino acid sequences and receptor interactions. The table below highlights some important protein families and growth factor groups included in this classification.
| Growth Factor Family | Growth Factors |
|
Fibroblast Growth Factor (FGF) |
|
|
Transforming Growth Factors |
|
|
Epidermal Growth Factor (EGF) |
- |
|
Insulin Family of Growth Factors |
|
|
Vascular Endothelial Growth Factor (VEGF) |
- |
|
Platelet-Derived Growth Factor (PDGF) |
|
|
Neural Growth Factors |
|
|
Growth Factors in blood |
|
|
Other key Growth Factors |
|
Understanding the Role of Different types of Growth Factors
Fibroblast Growth Factors
Fibroblast Growth Factors (FGFs) are small proteins that bind to specific receptor tyrosine kinases, known as Fibroblast Growth Factor Receptors (FGFRs).
FGFs are mainly produced by Macrophages. Other cells that secrete FGFs include fibroblasts, endothelial cells and epithelial cells. The functions of FGFs are diverse and context-dependent. They play crucial roles in embryonic development, tissue repair, angiogenesis, and the regulation of various physiological processes. The paracrine and autocrine actions of FGFs enable them to act in a localized manner, influencing nearby cells or the cells producing them.
FGFRs are transmembrane proteins expressed on the cell surface, and upon ligand binding, they activate downstream signaling pathways involved in cell proliferation, differentiation, migration, and survival. Upon binding to FGFRs, FGFs activate several signaling pathways, notably the Ras/MAPK pathway and the PI3K/Akt pathway. These pathways regulate gene expression, cell cycle progression, and cell survival, thereby impacting cellular behaviors and tissue homeostasis. Furthermore, FGF signaling is involved in intricate crosstalk with other signaling pathways, such as Wnt and BMP signaling, further modulating cellular responses.
Fig 2. Fibroblast Growth Factor 8 (isoform b) (Green) in complex with Fibroblast Growth Factor Receptor 2 (Blue). Source : PDB
Examples of FGFs include FGF-2, FGF-7, and FGF-23. FGF-2, also known as basic FGF (bFGF), is involved in angiogenesis, wound healing, and tissue regeneration. FGF-7, also known as keratinocyte growth factor (KGF), primarily acts on epithelial cells and plays a crucial role in skin and lung development and repair. FGF-23 is a hormone that regulates phosphate homeostasis and bone mineralization.
Transforming Growth Factor-beta (TGF-β) family
The Transforming Growth Factor-beta (TGF-β) family comprises a group of evolutionarily conserved cytokines involved in a wide range of biological processes. This family includes three major isoforms, TGF-β1, TGF-β2, and TGF-β3, each encoded by distinct genes. Additionally, other members of the TGF-β family include factors such as Activins, Inhibins, and Growth and Differentiation Factors (GDFs).
Members of the TGF-β family exert their effects by binding to TGF-β receptors. These receptors are serine/threonine kinases, consisting of type I and type II receptors. Upon ligand binding, the type II receptor phosphorylates and activates the type I receptor, initiating intracellular signaling pathways.
Fig. 3 Crystal Structure of TGF-beta 3 . Source: PDB
TGF-β family members are produced by various cell types, including immune cells, fibroblasts, and epithelial cells. They are involved in diverse biological processes, such as embryonic development, tissue homeostasis, immune regulation, and wound healing. Furthermore, dysregulation of TGF-β family signaling has been implicated in several diseases, including cancer, fibrosis, and developmental disorders.
The canonical signaling pathway of the TGF-β family involves activation of the Smad proteins. Upon receptor activation, Smad proteins are phosphorylated and form complexes that translocate into the nucleus, where they modulate the expression of target genes. In addition to the Smad pathway, TGF-β family members can also activate non-canonical signaling pathways, including MAPK and PI3K/Akt, to exert their effects on cellular responses.
Examples of the TGF-β family members include:
- TGF-β1: It is involved in regulating immune responses, tissue remodeling, and wound healing. Dysregulation of TGF-β1 signaling has been associated with fibrosis and cancer progression.
- TGF-β2: It plays a critical role in embryonic development, particularly in the development of organs such as the heart and lungs. TGF-β2 also contributes to tissue repair and regulation of immune responses.
- TGF-β3: It is essential for proper development and morphogenesis of various tissues, including the palate and skin. TGF-β3 is involved in regulating cell proliferation, differentiation, and apoptosis during development.
- Growth Differentiation Factor-9 (GDF-9): GDF-9 is a member of the transforming growth factor-beta (TGF-β) superfamily. It plays a critical role in the growth and differentiation of various cell types, particularly in reproductive tissues. GDF-9 is primarily produced in the ovaries and is involved in follicle development, oocyte maturation, and fertility regulation.
Epidermal Growth Factor
Fig 4. Crystal Structure of Human Epidermal Growth Factor. Source: PDB
Epidermal Growth Factor (EGF) is a polypeptide growth factor that plays a significant role in cell growth, proliferation, and differentiation. It is produced by various cell types, including fibroblasts, macrophages, and salivary glands. EGF binds to and activates the EGF receptor (EGFR), a transmembrane receptor tyrosine kinase, initiating a cascade of intracellular signaling events.
Upon EGF binding, EGFR undergoes dimerization and autophosphorylation, leading to the activation of downstream signaling pathways like the Ras-Raf-MAPK pathway and the PI3K-Akt pathway. EGF exerts its effects on several cell types, including epidermal cells, fibroblasts, and various epithelial cells. In the skin, EGF promotes cell proliferation, migration, and wound healing. It stimulates the production of collagen and elastin, essential components of the extracellular matrix, and contributes to tissue regeneration and repair.
Beyond skin cells, EGF also influences the growth and development of various other tissues and organs. It plays a role in embryonic development, promoting organ formation and tissue differentiation. EGF is involved in the growth and maintenance of the gastrointestinal epithelium, where it stimulates the proliferation of intestinal cells and contributes to tissue homeostasis.
Epidermal-Like Growth Factors
Epidermal-like growth factors, share structural and functional similarities with EGF. They also bind to the Epidermal Growth Factor Receptor (EGFR) or related receptors. Upon binding, they activate similar downstream signaling pathways, including the Ras/MAPK and PI3K/Akt pathways. They are produced by various cell types, including epithelial cells, fibroblasts, and immune cells. Epidermal-like growth factors contribute to tissue development, wound healing, and immune responses.
Examples of Epidermal-like Growth Factors:
- Amphiregulin (AREG): AREG is produced in various tissues, including the skin, lungs, and intestine. It binds to EGFR and activates signaling pathways involved in tissue repair, inflammation, and cancer progression. It is thought to play a major role in development and maturation of placenta
- Betacellulin (BTC): BTC is produced in several tissues, including the pancreas and mammary glands. It binds to EGFR and other ErbB receptors, regulating cell proliferation, differentiation, and insulin secretion.
- Epiregulin (EREG): EREG is produced in various tissues, including the skin and gastrointestinal tract. It binds to EGFR and triggers signaling cascades that contribute to wound healing, cell proliferation, and tissue regeneration.
Insulin Family of Growth Factors
Insulin and Insulin-like Growth Factors (IGFs) are peptide hormones that play crucial roles in regulating growth, metabolism, and cellular functions. They are part of the insulin family of growth factors and share structural and functional similarities. Insulin and IGFs exert their effects by binding to specific cell surface receptors and activating intracellular signaling pathways.
Insulin is primarily produced by pancreatic beta cells in response to elevated blood glucose levels. It acts as a key regulator of carbohydrate, lipid, and protein metabolism. Insulin binds to the Insulin Receptor (IR), a receptor tyrosine kinase present on various cell types, including liver, muscle, and adipose tissue. Upon binding, insulin triggers a cascade of intracellular signaling events, leading to the uptake of glucose into cells, storage of glucose as glycogen, and the synthesis of proteins and lipids.
Fig 5. Crystal Structure of Insulin-like Growth Factor -1. Source : PDB
Insulin-like Growth Factors (IGFs), including IGF-1 and IGF-2, are mainly produced in the liver, although other tissues can also produce them. IGF-1 is a key mediator of growth hormone action and plays a vital role in promoting cell growth, proliferation, and differentiation. IGF-2 is involved in embryonic development and tissue growth. Both IGF-1 and IGF-2 bind to the IGF Receptor (IGFR), which is structurally similar to the IR. Activation of IGFR initiates signaling cascades that regulate cell survival, growth, and metabolism.
The signaling pathways activated by insulin and IGFs involve the phosphorylation of intracellular substrates, including Insulin Receptor Substrate (IRS) proteins, which transmit the signals to downstream effectors. The main pathways include the PI3K/Akt pathway, which regulates cell growth, survival, and metabolism, and the Ras/MAPK pathway, which is involved in cell proliferation and differentiation.
Insulin and IGFs collectively influence numerous physiological processes, such as regulating glucose homeostasis, promoting cell growth and differentiation, and modulating metabolism. Dysregulation of insulin and IGF signaling is associated with metabolic disorders like diabetes and growth disorders like acromegaly and dwarfism.
Vascular Endothelial Growth Factor (VEGF)
Fig 6. Structure of VEGF-D. Source: PDB
Vascular Endothelial Growth Factor (VEGF) is a key regulator of angiogenesis, the formation of new blood vessels from pre-existing ones. It is a cytokine that plays a critical role in various physiological and pathological processes, particularly in the development of blood vessels, tissue repair, and tumor angiogenesis.
VEGF is produced by various cell types, including endothelial cells, macrophages, and certain tumor cells. It acts by binding to specific receptors on the surface of endothelial cells, primarily VEGF Receptor 1 (VEGFR1) and VEGF Receptor 2 (VEGFR2). Upon ligand binding, VEGFRs activate intracellular signaling cascades, leading to endothelial cell proliferation, migration, and the formation of new blood vessels.
The functions of VEGF extend beyond angiogenesis. It also promotes vascular permeability, allowing the exchange of nutrients, oxygen, and immune cells between blood vessels and surrounding tissues. Moreover, VEGF is involved in lymphangiogenesis, the formation of lymphatic vessels, which play a crucial role in immune response and fluid drainage.
The signaling pathways activated by VEGF primarily involve the PI3K/Akt and MAPK/ERK pathways. These pathways regulate endothelial cell survival, proliferation, migration, and the expression of genes associated with angiogenesis. Additionally, VEGF signaling interacts with other signaling pathways, such as Notch and Wnt, to fine-tune angiogenic responses.
VEGF's role in angiogenesis is of great significance in both physiological and pathological conditions. During development, VEGF guides the formation of blood vessels, ensuring proper tissue growth and organ development. In adults, VEGF is involved in tissue repair, wound healing, and the restoration of blood flow after ischemic events. However, dysregulated VEGF signaling is implicated in various diseases, including cancer, diabetic retinopathy, and age-related macular degeneration.
Examples of VEGF isoforms include VEGF-A, VEGF-B, VEGF-C, and VEGF-D, each with distinct functions and receptor specificities. VEGF-A is the most extensively studied isoform and is involved in promoting angiogenesis and vascular permeability. VEGF-C and VEGF-D are primarily involved in lymphangiogenesis.
Platelet-Derived Growth Factors (PDGF)
Structure of PDGF-BB homodimer. Source: PDB
Platelet-Derived Growth Factor (PDGF) is a potent growth factor that is released by platelets, as well as various other cell types, including smooth muscle cells, fibroblasts, and macrophages. There are different types of PDGF forms like AA, BB and AB
PDGF exerts its effects by binding to specific cell surface receptors known as PDGF receptors (PDGFRs), which are receptor tyrosine kinases. There are two main types of PDGFRs, namely PDGFR- alpha and PDGFR-beta. Upon binding to PDGFRs, PDGF activates downstream signaling pathways like Ras/MAPK pathway, which regulates cell proliferation and differentiation, and the PI3K/Akt pathway, which is involved in cell survival and migration.
PDGF plays a significant role in tissue repair and wound healing. It stimulates the recruitment and proliferation of fibroblasts, which are responsible for synthesizing and depositing extracellular matrix components. PDGF also promotes the migration and proliferation of smooth muscle cells, contributing to the repair and remodeling of blood vessels. Additionally, PDGF regulates the proliferation and survival of certain cell types during embryonic development.
PDGF is associated with various diseases and pathological conditions. Dysregulation of PDGF signaling has been implicated in fibrotic disorders, such as pulmonary fibrosis and liver fibrosis, where excessive PDGF signaling contributes to aberrant tissue remodeling. PDGF is also involved in the development and progression of certain types of cancers, where it promotes cell proliferation, angiogenesis, and metastasis.
Neural Growth Factors
Neural Growth Factors are a group of proteins that play essential roles in the development, survival, and maintenance of neurons in the nervous system. They are key regulators of neural growth, differentiation, and synaptic plasticity. Several types of neural growth factors have been identified, each with unique functions and specificities.
One prominent neural growth factor is Nerve Growth Factor (NGF). NGF is crucial for the survival and maintenance of sympathetic and sensory neurons. It is produced by various cell types, including target tissues, immune cells, and glial cells. NGF binds to its specific receptor, the TrkA receptor, initiating signaling cascades that promote neuronal survival, neurite outgrowth, and the formation of functional connections.
Crystal Structure of Beta Nerve growth Factor
Another important neural growth factor is Brain-Derived Neurotrophic Factor (BDNF). BDNF is widely distributed in the brain and is involved in promoting the growth, differentiation, and survival of various neuronal populations. It acts through the TrkB receptor and plays a critical role in regulating synaptic plasticity, neuronal connectivity, and learning and memory processes.
Other neural growth factors include Neurotrophin-3 (NT-3) and Neurotrophin-4/5 (NT-4/5). These growth factors support the survival and development of specific subsets of neurons during embryonic and early postnatal stages. They bind to various Trk receptors and are involved in promoting axonal growth, dendritic arborization, and synapse formation.
The signaling pathways activated by neural growth factors involve the activation of receptor tyrosine kinases, such as the Trk receptors, and subsequent activation of downstream signaling cascades, including the PI3K/Akt and MAPK/ERK pathways. These pathways regulate gene expression, cytoskeletal dynamics, and cellular processes critical for neuronal growth, survival, and synaptic plasticity.
Neural growth factors are vital for the development and maintenance of the nervous system. They contribute to the establishment of proper neuronal connections during development and support the survival and function of neurons throughout life. Dysregulation of neural growth factor signaling has been associated with various neurological disorders, including neurodegenerative diseases and psychiatric disorders.
Growth Factors in Blood
- Colony Stimulating Factors (CSFs): CSFs are a group of growth factors that regulate the production, differentiation, and function of hematopoietic stem cells and progenitor cells in the bone marrow. They include granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), and macrophage colony-stimulating factor (M-CSF). CSFs stimulate the production of specific blood cell types, such as granulocytes, macrophages, and red blood cells.
- Interleukins (ILs): Interleukins are a group of cytokines that function as growth factors, primarily involved in immune regulation and inflammation. Several interleukins have growth-promoting effects on specific cell types. For example, IL-2 is known as T cell growth factor as it stimulates the growth and proliferation of T lymphocytes, playing a vital role in immune responses. Other interleukins, such as IL-3, IL-4, and IL-7, also have growth-promoting effects on various immune cells.
- Erythropoietin (EPO) is a kidney-produced glycoprotein growth factor that regulates red blood cell production. It stimulates the proliferation, differentiation, and survival of erythroid progenitor cells in the bone marrow. By binding to specific receptors, EPO activates signaling pathways that enhance red blood cell formation. EPO is primarily involved in responding to low oxygen levels in the body and plays a vital role in maintaining oxygen transport. It also exhibits tissue-protective properties and has therapeutic potential in conditions such as chronic kidney disease and neurodegenerative disorders. Clinically, EPO and its analogs are used to treat anemia associated with various medical conditions.
Other Key Growth Factors
1. Hepatocyte Growth Factor (HGF): HGF is a growth factor primarily produced by mesenchymal cells. It acts on hepatocytes (liver cells) and plays a key role in liver regeneration, tissue repair, and development. HGF promotes cell growth, survival, and migration, contributing to liver tissue maintenance and regeneration after injury.
2. Hepatoma-Derived Growth Factor (HDGF): HDGF is a growth factor initially identified in hepatoma cells. It is involved in various cellular processes, including cell proliferation, survival, and migration. HDGF plays a role in tissue repair, angiogenesis, and embryonic development. It is also implicated in cancer progression and metastasis.
3. Migration Stimulating Factor (MSF): MSF, is a growth factor that promotes cell migration. It is structurally related to HGF and shares some functional similarities. MSF/HGFL stimulates cell motility and migration in a variety of cell types and contributes to tissue repair and regeneration.
Related Products

| Human NRG-3 / Neuregulin 3 ELISA Kit | |
|---|---|
| ELISA TYPE: | Sandwich ELISA, Double Antibody |
| SENSITIVITY: | 9.375pg/ml |
| RANGE: | 15.625-1000pg/ml |
The human Neuregulin 3 (NRG3) ELISA kit enables accurate and quantitative measurement of NRG3 protein levels in biological samples. It is a valuable tool for studying NRG3's role in physiological processes, such as neural development, synaptic plasticity, and neurotransmission.

| Placental Growth Factor ELISA Kit | |
|---|---|
| ELISA TYPE: | Sandwich |
| SENSITIVITY: | 9.38pg/mL |
| RANGE: | 15.63-1000pg/mL |
The Placental Growth Factor (PlGF) ELISA kit is a reliable tool for quantifying PlGF levels in biological samples, enabling researchers to study its role in pregnancy, angiogenesis, and various diseases. The kit offers precise measurement, facilitating the investigation of PlGF as a potential biomarker and therapeutic target in diverse clinical settings.
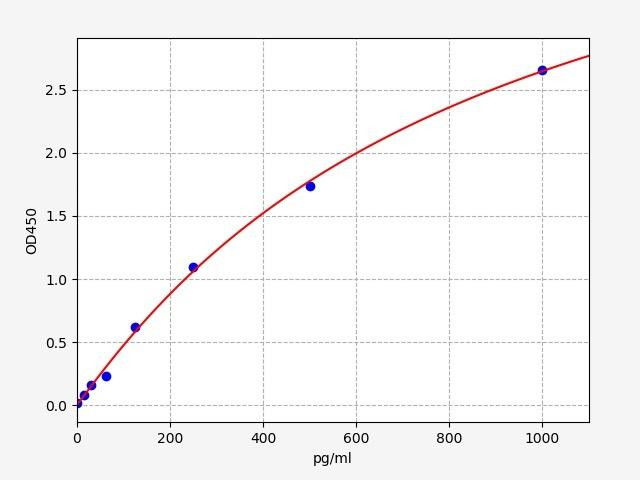
| Human TNF alpha ELISA | |
|---|---|
| ELISA TYPE: | Sandwich ELISA, Double Antibody |
| SENSITIVITY: | 9.375pg/ml |
| RANGE: | 15.625-1000pg/ml |
The Human Tumor Necrosis Factor alpha (TNF-alpha) ELISA kit is a valuable tool for accurately quantifying TNF-alpha levels in biological samples. It enables researchers to explore the role of TNF-alpha in inflammation, immune response, and understand its involvement in pathogenesis in order to facilitate its potential as a biomarker and therapeutic target in clinical research.
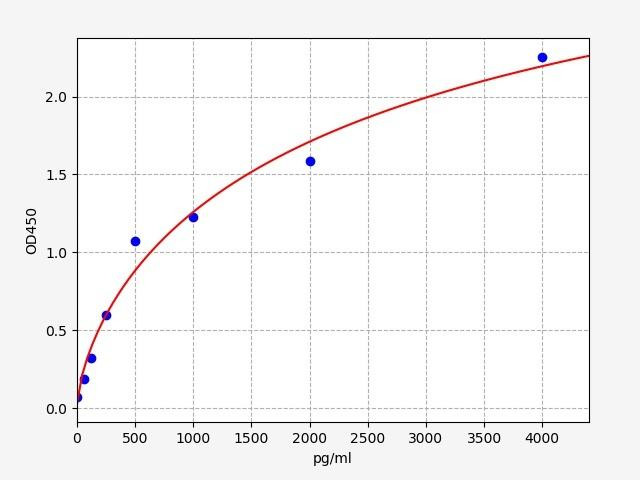
| Human GDF7 ELISA Kit | |
|---|---|
| ELISA TYPE: | Sandwich ELISA, Double Antibody |
| SENSITIVITY: | 37.5pg/ml |
| RANGE: | 62.5-4000pg/ml |
The Human Growth Differentiation Factor 7 (GDF-7) ELISA kit is a valuable tool for precise quantification of GDF-7 levels in biological samples. It enables researchers to explore the role of GDF-7 in developmental processes, tissue regeneration, and disease pathogenesis.
In conclusion, growth factors are indispensable players in biological processes, exerting profound effects on cell growth, differentiation, and tissue development. Their intricate signaling networks and diverse functions make them key targets of scientific investigation, offering new avenues for understanding cellular mechanisms and potential therapeutic interventions. Through extensive research and the development of advanced technologies like ELISA, we continue to unveil the complex roles of growth factors in health and disease. As our knowledge expands, harnessing the potential of growth factors holds great promise for unlocking novel treatments and improving human well-being.
Written by Rithika Suresh
Rithika Suresh completed her undergraduate degree in Biotechnology in Anna University before completing her masters in Biotechnology at University College Dublin.
Recent Posts
-
The Rise of Alpha-Emitting Radiopharmaceuticals: A New Era in Targeted Cancer Therapy
Imagine a microscopic sniper, capable of delivering a lethal blow to a single cancer cell while lea …19th Feb 2026 -
Targeted Protein Degradation: The Next Frontier in Oncology Drug Discovery
For decades, cancer drug development has focused on blocking proteins—inhibiting enzymes, antagoniz …18th Feb 2026 -
Neuroinflammation and Compulsive Behavior: The Role of Astrocytes in OCD
Imagine a mind trapped in a loop, endlessly repeating actions or thoughts, driven by an invisible f …18th Feb 2026