The role of IL-18 in health and disease
Exploring IL-18: A key cytokine in inflammation and disease.
Key Takeaways:
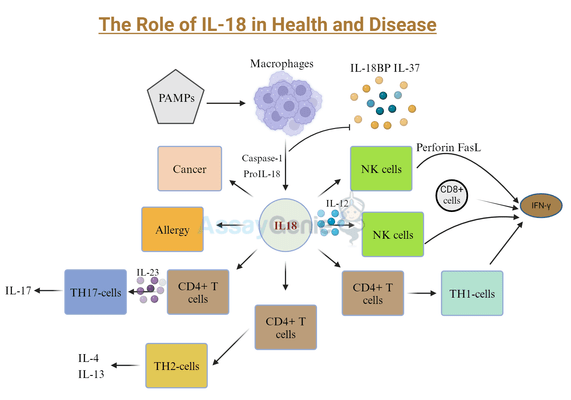
- IL-18, a pro-inflammatory cytokine, plays a pivotal role in immune responses, particularly in inducing Type II interferon and activating macrophages.
- It's produced as an inactive form and activated by caspase-1, signaling through IL-18 receptors to trigger inflammation.
- IL-18's regulation involves IL-18 binding protein (IL-18BP) and IL-37, which modulate its activity and prevent excessive inflammation.
- It contributes to T cell differentiation and NK cell cytotoxicity, influencing various immune functions.
- IL-18 is implicated in several diseases, including Crohn's disease and sepsis, making it a potential biomarker for these conditions.
IL-18 Overview
Interleukin-18 (IL-18), located on chromosome 11 in humans, encodes a 24 kDa polypeptide that was first described in 1989, and is a pro-inflammatorycytokine which induces Type II interferon (IFNg) secretion (Dinarello et al, 2013; Tomura et al, 1998; Nakamura et al, 1989). In 1995, the first cloning of IL-18 took place, along with the name change from IFNg-inducing factor to IL-18, allowing for further analysis of its structure which was found to be similar to IL-1, with human IL-18 and IL-1b sharing a b-pleated structure and exploit the same signaling pathways (Tsutsui et al, 1997; Okamura et al, 1995). Along with IL-1, IL-18 was discovered to be a key mediator of the inflammatory response. Additionally, IL-18 has multiple other functions including the induction of macrophage activation (Giacomini et al, 2001), the maturation of Th1CD4+ T cells (Xu et al, 2000), promoting angiogenesis (Park et al, 2001), and sensitizing cells to apoptosis by increasing the lymphocyte-specific expression of Fas ligand (FasL) (Hashimoto et al, 1999), a potent death receptor agonist.
IL-18 Signalling
One of the main similarities between IL-1b and IL-18 is the caspase-1 mediated cleavage of IL-18 in the cytosol, rendering the active 18 kDa form of IL-18 (Ghayur et al, 1997). IL-18 can also be released from cells via pyroptosis, a form of cell death induced by caspase-1 and inflammasome activation. Extracellular IL-18 binds to the membranous a-chain of the IL-18 receptor, forming a complex, however this binding occurs at a relatively low affinity. A higher affinity binding occurs upon ligation of IL-18 with the b-chain of the IL-18 receptor, forming a heterodimeric complex of IL-18, IL-18Ra and IL-18Rb, which leads to the induction of intracellular signalling (Hoshino et al, 1999; Torigoe et al, 1997). Expression of IL-18Ra is ubiquitous, however IL-18Rb expression is more limited and reserved primarily for T cells, macrophages, dendritic cells and some subsets of endothelial cells (Gerdes et al, 2002). Upon receptor activation, the Toll-IL-1 receptor (TIR) domain proteins form a complex at the membrane and downstream signalling through interleukin-1 receptor associated kinases 1,2 and 4 (IRAKs 1,2 and 4) and tumour necrosis factor receptor associated factor 6 (TRAF6), and subsequent degradation of the cytoplasmic inhibitory components of NF-kB, culminating in NF-kB translocation to the nucleus and activation (Akira, 2000; Adachi et a, 1998). IL-18 can also signal to STAT3, inducing its phosphorylation, and also mediate the induction of MAPK signalling (Wyman et al, 2002; Kalina et al, 2000).
Regulation of IL-18
IL-18 binding protein (IL-18BP) is molecule that is constitutively secreted, and, as the name suggests, binds to and neutralizes IL-18, preventing the ligation of IL-18 with IL-18R, thus downregulating IFNg induction. IL-18BP therefore regulates the Th1 response of the immune system, and in infectious pathogeneses the levels of IL-18BP in the serum of patients are not sufficient to neutralize the pro-inflammatory effects of IL-18 (Novik et al, 2001). IL-18BP transcription is under the control of IFNg and therefore acts in a negative feedback loop to regulate IL-18 signalling (Muhl et al, 2000).
Additionally, IL-37 is a secreted cytokine that acts as an inhibitor of the innate immune response by binding to IL-18Ra, but not IL-18Rb, effectively competing with IL-18 for receptor binding (Nold et al, 2010). The mechanism of action of IL-37 is demonstrated to occur via the binding of IL-37 to a complex including IL-18Ra and IL-1R8 (also known as SIGGR) to induce anti-inflammatory responses, thereby combating the effects of IL-18 (Li et al, 2015). Interestingly, IL-18BP also binds to IL-37 and limits its anti-inflammatory effects (Banda et al, 2003).
Diverse biological functions of IL-18
A unique characteristic of IL-18 is the role in the differentiation and maturation of T cells. IL-18, in combination with fellow cytokine IL-12, induces IFNg production in CD4+ and CD8+ single positive T cells, as well as natural killer (NK) cells and macrophages (Nakanishi et al, 2001; Munder et al, 1998). IFNg production occurs via the concurrent IL-18 – dependent stimulation of STAT4 and IL-12 – dependent stimulation of NF-kB (Nakanishi et al, 2001). In the absence of IL-12, IL-18 does not induce IFNg, but instead promotes the differentiation of T cells in Th2 cells (Yoshimoto et al, 2000). IL-18 may negatively regulate Th17 T cells differentiation (Hitzler et al, 2012), however throughout the literature to date this specific role remains unclear.
Furthermore, IL-18 induces the cytotoxicity of NK cells in a FasL-dependent manner (Tsutsui et al, 1996). The ability of IL-18 to upregulate FasL may mediate severe pathogenic conditions, such as fever (Gatti et al, 2002). In addition to the IFNg – centric functions of IL-18, an increase in nitric oxide synthesis, increased cell adhesion molecules and elevated levels of chemokines are all described in response to IL-18 stimulation (Morel et al, 2001; Kohka et al, 1998).
Pathogenesis of IL-18
IL-18 has been identified to have a role in multiple infectious, metabolic or inflammatory diseases, such as Crohn’s disease, inflammatory bowel disease, influenza virus and chronic obstructive pulmonary disease (COPD) (Imaoka et al, 2008; Naftali et al, 2007; Saraneva et al, 1998). Additionally, elevated levels of IL-18 in post-operative patients are indicative of sepsis pathogenesis (Emmanuilidis et al, 2002). Under homeostatic conditions in the gut, IL-18 is produced by endothelial cells, facilitating the maintenance of a healthy microbiota. However, pathogen-induced secretion of IL-18 from macrophages occurs upon disruption of the intestinal barrier in inflammatory bowel disease and initiates a robust pro-inflammatory response (Lissner et al, 2015). As IL-18 has many diverse physiological functions, targeting IL-18 for use as a therapeutic currently is limited, however, it has proven to be a valid biomarker for multiple pathogeneses.
References
- Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998. 9(1):143-50.
- Akira S. The role of IL-18 in innate immunity. Curr Opin Immunol. 2000. 12(1):59-63.
- Banda NK, Vondracek A, Kraus D, Dinarello CA, Kim SH, Bendele A, Senaldi G, Arend WP. Mechanisms of inhibition of collagen-induced arthritis by murine IL-18 binding protein. J Immunol. 2003. 170(4):2100-5.
- Dinarello CA, Novick D, Kim S, Kaplanski G. Interleukin-18 and IL-18 binding protein. Front Immunol. 2013. 4:289.
- Emmanuilidis K, Weighardt H, Matevossian E, Heidecke CD, Ulm K, Bartels H, Siewert JR, Holzmann B. Differential regulation of systemic IL-18 and IL-12 release during postoperative sepsis: high serum IL-18 as an early predictive indicator of lethal outcome. Shock. 2002. 18(4):301-5.
- Gatti S, Beck J, Fantuzzi G, Bartfai T, Dinarello CA. Effect of interleukin-18 on mouse core body temperature. Am J Physiol Regul Integr Comp Physiol. 2002. 282(3):R702-9.
- Gerdes N, Sukhova GK, Libby P, Reynolds RS, Young JL, Schönbeck U. Expression of interleukin (IL)-18 and functional IL-18 receptor on human vascular endothelial cells, smooth muscle cells, and macrophages: implications for atherogenesis. J Exp Med. 2002. 195(2):245-57.
- Ghayur T, Banerjee S, Hugunin M, Butler D, Herzog L, Carter A, Quintal L, Sekut L, Talanian R, Paskind M, Wong W, Kamen R, Tracey D, Allen H. Caspase-1 processes IFN-gamma-inducing factor and regulates LPS-induced IFN-gamma production. Nature. 1997. 386(6625):619-23.
- Giacomini E, Iona E, Ferroni L, Miettinen M, Fattorini L, Orefici G, Julkunen I, Coccia EM. Infection of human macrophages and dendritic cells with Mycobacterium tuberculosis induces a differential cytokine gene expression that modulates T cell response. J Immunol. 2001. 166(12):7033-41.
- Hashimoto W, Osaki T, Okamura H, Robbins PD, Kurimoto M, Nagata S, Lotze MT, Tahara H. Differential antitumor effects of administration of recombinant IL-18 or recombinant IL-12 are mediated primarily by Fas-Fas ligand- and perforin-induced tumor apoptosis, respectively. J Immunol. 1999. 163(2):583-9.
- Hitzler I, Sayi A, Kohler E, Engler DB, Koch KN, Hardt WD, Müller A. Caspase-1 has both proinflammatory and regulatory properties in Helicobacter infections, which are differentially mediated by its substrates IL-1β and IL-18. J Immunol. 2012. 188(8):3594-602.
- Imaoka H, Hoshino T, Takei S, Kinoshita T, Okamoto M, Kawayama T, Kato S, Iwasaki H, Watanabe K, Aizawa H. Interleukin-18 production and pulmonary function in COPD. Eur Respir J. 2008. 31(2):287-97.
- Kalina U, Kauschat D, Koyama N, Nuernberger H, Ballas K, Koschmieder S, Bug G, Hofmann WK, Hoelzer D, Ottmann OG. IL-18 activates STAT3 in the natural killer cell line 92, augments cytotoxic activity, and mediates IFN-gamma production by the stress kinase p38 and by the extracellular regulated kinases p44erk-1 and p42erk-21. J Immunol. 2000. 165(3):1307-13.
- Kohka H, Yoshino T, Iwagaki H, Sakuma I, Tanimoto T, Matsuo Y, Kurimoto M, Orita K, Akagi T, Tanaka N. Interleukin-18/interferon-gamma-inducing factor, a novel cytokine, up-regulates ICAM-1 (CD54) expression in KG-1 cells. J Leukoc Biol. 1998. 64(4):519-27.
- Li S, Neff CP, Barber K, Hong J, Luo Y, Azam T, Palmer BE, Fujita M, Garlanda C, Mantovani A, Kim S, Dinarello CA. Extracellular forms of IL-37 inhibit innate inflammation in vitro and in vivo but require the IL-1 family decoy receptor IL-1R8. Proc Natl Acad Sci U S A. 2015. 112(8):2497-502.
- Lissner D, Schumann M, Batra A, Kredel LI, Kühl AA, Erben U, May C, Schulzke JD, Siegmund B. Monocyte and M1 Macrophage induced Barrier Defect Contributes to Chronic IntestinalInflammation in IBD. Inflamm Bowel Dis. 2015. 21(6):1297-305.
- Morel JC, Park CC, Woods JM, Koch AE. A novel role for interleukin-18 in adhesion molecule induction through NF kappa B and phosphatidylinositol (PI) 3-kinase-dependent signal transduction pathways. J Biol Chem. 2001. 276(40):37069-75.
- Mühl H, Kämpfer H, Bosmann M, Frank S, Radeke H, Pfeilschifter J. Interferon-gamma mediates gene expression of IL-18 binding protein in nonleukocytic cells. Biochem Biophys Res Commun. 2000. 267(3):960-3.
- Munder M, Mallo M, Eichmann K, Modolell M. Murine macrophages secrete interferon gamma upon combined stimulation with interleukin (IL)-12 and IL-18: A novel pathway of autocrine macrophage activation. J Exp Med. 1998. 187(12):2103-8.
- Naftali T, Novick D, Gabay G, Rubinstein M, Novis B. Interleukin-18 and its binding protein in patients with inflammatory bowel disease during remission and exacerbation. Isr Med Assoc J. 2007. 9(7):504-8.
- Nakamura K, Okamura H, Wada M, Nagata K, Tamura T. Endotoxin induced serum factor that stimulates gamma interferon production. Infect Immun. 1989. 57(2):590-5.
- Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 regulates both Th1 and Th2 responses. Annu Rev Immunol. 2001. 19:423-74.
- Nold MF, Nold-Petry CA, Zepp JA, Palmer BE, Bufler P, Dinarello CA. IL-37 is a fundamental inhibitor of innate immunity. Nat Immunol. 2010. 11(11):1014-22.
- Novick D, Schwartsburd B, Pinkus R, Suissa D, Belzer I, Sthoeger Z, Keane WF, Chvatchko Y, Kim SH, Fantuzzi G, Dinarello CA, Rubinstein M. A novel IL-18BP ELISA shows elevated serum IL-18BP in sepsis and extensive decrease of free IL-18. Cytokine. 2001 Jun 21;14(6):334-42.
- Okamura H, Tsutsi H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K, et al. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 1995. 378(6552):88-91.
- Park CC, Morel JC, Amin MA, Connors MA, Harlow LA, Koch AE. Evidence of IL-18 as a novel angiogenic mediator. J Immunol. 2001. 167(3):1644-53.
- Sareneva T, Matikainen S, Kurimoto M, Julkunen I. Influenza A virus-induced IFN-alpha/beta and IL-18 synergistically enhance IFN-gamma gene expression in human T cells. J Immunol. 1998. 160(12):6032-8.
- Tomura M, Zhou XY, Maruo S, Ahn HJ, Hamaoka T, Okamura H, Nakanishi K, Tanimoto T, Kurimoto M, Fujiwara H. A critical role for IL-18 in the proliferation and activation of NK1.1+ CD3- cells. J Immunol. 1998. 160(10):4738-46.
- Torigoe K, Ushio S, Okura T, Kobayashi S, Taniai M, Kunikata T, Murakami T, Sanou O, Kojima H, Fujii M, Ohta T, Ikeda M, Ikegami H, Kurimoto M. Purification and characterization of the human interleukin-18 receptor. J Biol Chem. 1997. 272(41):25737-42.
- Tsutsui H, Nakanishi K, Matsui K, Higashino K, Okamura H, Miyazawa Y, Kaneda K. IFN-gamma-inducing factor up-regulates Fas ligand-mediated cytotoxic activity of murine natural killer cell clones. J Immunol. 1996. 157(9):3967-73.
- Wyman TH, Dinarello CA, Banerjee A, Gamboni-Robertson F, Hiester AA, England KM, Kelher M, Silliman CC. Physiological levels of interleukin-18 stimulate multiple neutrophil functions through p38 MAP kinase activation. J Leukoc Biol. 2000. 72(2):401-9.
- Xu D, Trajkovic V, Hunter D, Leung BP, Schulz K, Gracie JA, McInnes IB, Liew FY. IL-18 induces the differentiation of Th1 or Th2 cells depending upon cytokine milieu and genetic background. Eur J Immunol. 2000. 30(11):3147-56.
- Yoshimoto T, Mizutani H, Tsutsui H, Noben-Trauth N, Yamanaka K, Tanaka M, Izumi S, Okamura H, Paul WE, Nakanishi K. IL-18 induction of IgE: dependence on CD4+ T cells, IL-4 and STAT6. Nat Immunol. 2000. 1(2):132-7.
Related tools for research
Related Immunology Content
- Adhesion Molecules in Atherosclerosis - ICAM1
- Adaptive Immunity
- B Cells
- Brown Fat Macrophages
- Carbon Dioxide Signalling in Immune Cells
- Cortisol and the immune response
- Chemokines & Chemokine Receptors
- Dendritic Cells
- Immunometabolism Assays
- Inflammation & Aging Review
- Inflammation & Obesity Review
- Glycolysis Assay Kits
- Heterogeneity of Type 1 diabetes in children
- Macrophages
- Neutophils
- Natural Killer (NK) Cells
- Natural Killer Cells & Metabolism Review
- NLRP3 Inflammasome
- Mononuclear Phagocytes Review
- Multiple Sclerosis and Stem Cells
- Platelet reactivity & Diet Review
- SOCS proteins review
- TCA assay Kits
- T Cell assay types
- T Cells & Acute Leukemia Review
- T Cells & Hepatitis Review
- T Cell Metabolism
- T Cell responses in Diabetes
- TNF alpha & Inflammation
- TLR mediated Inflammation Review
- TLR Signalling & Neurodegeneration Review
- Trauma Immunology
- Wnt Signalling Pathway in Immunity
- What is Sepsis?
Recent Posts
-
IgG1 Plasma Cells: The Emerging Biomarker for Predicting Cancer Immunotherapy Success
In the relentless fight against cancer, immunotherapy has emerged as a beacon of hope, harnessing t …24th Feb 2026 -
The Rise of Cancer Neuroscience: How Neural Circuits Drive Tumor Progression
For decades, we viewed cancer as a rogue army of cells, a biological glitch driven solely by geneti …23rd Feb 2026 -
CRISPR-Powered Light Sensors: A New Frontier in Ultra-Sensitive Cancer Detection
Cancer detection often relies on advanced imaging or invasive procedures, frequently catching the d …20th Feb 2026